Abstract
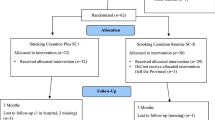
Background: Interventions for smoking cessation have been typically evaluated on reactively recruited samples in clinical trials (efficacy trials). However, to have an impact on smoking rates in a general population, the intervention should also be evaluated with proactively recruited representative samples (effectiveness trials).Purpose: The characteristics of participants and two groups of nonparticipants recruited for a population-based nicotine replacement therapy study were compared.Methods: All members of a large New England Veterans’ Administration Medical Center were contacted, and interviews were completed with 3,239 identified smokers (at least 10 cigarettes per day). At the end of the interview, all smokers were offered participation in a multiple intervention study. Of the interviewed smokers, 2,915 verbally agreed to participate in the study (90%). Of those who gave initial verbal consent, 2,054 returned the written informed consent form and became participants (70%).Results: The participants (full consent group) differed significantly from both nonparticipant groups—that is, the smokers who were interviewed but declined participation by active refusal (survey only group) and those who gave verbal consent but passively refused participation by failing to return the written consent form (verbal consent only group). Participants were more likely to be married, younger, and female; to live with others; and to have previously used or considered using nicotine replacement therapy. The survey only group was also more likely to be in the precontemplation stage (54%), whereas the participants were more likely to be in the contemplation (46%) or preparation stage (35%). The verbal consent only group was intermediate of the other two groups in stage-of-change characteristics.Conclusions: An important finding was that it is possible to recruit a large proportion of a sample of identified smokers to an nicotine replacement therapy study. However, the participants are likely to differ in significant ways from those who either actively or passively decline participation.
Similar content being viewed by others
References
Petro R, Lopez A: World-wide mortality from current smoking patterns. In Durstone B, Jamrogik K (eds),The Global War: Proceedings of the Seventh World Conference on Tobacco and Health. East Perth, Western Australia:1990, 62–68.
Fiore MC, Smith SS, Jorenby DE, Baker TB: The effectiveness of the nicotine patch for smoking cessation: A meta-analysis.Journal of the American Medical Association. 1994,266:3139–3144.
Po ALW: Transdermal nicotine in smoking: A meta-analysis.European Journal of Clinical Pharmacology. 1993,45:519–528.
Silagy C, Mant D, Fowler G, Lodge M: Meta-analysis on effi-cacy of nicotine replacement therapies in smoking cessation.Lancet. 1994,343:139–142.
Tang JL, Law M, Wald N: How effective is nicotine replacement therapy in hel** people to stop smoking?British Medical Journal. 1994,308:21–26.
Hughes JR, Shiffman S, Callas P, Zhang J: A meta-analysis of the efficacy of over-the-counter nicotine replacement.Tobacco Control. 2003,12:21–27.
Pierce JP, Gilpin EA: Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation.Journal of the American Medical Association. 2002,288:1260–1264.
Flay BR: Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs.Preventive Medicine. 1986,15:451–474.
Glasgow RE, Lichtenstein E, Marcus AC: Why don we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition.American Journal of Public Health. 2003,93:1261–1267.
Greenwald P, Cullen JW: The new emphasis in cancer control.Journal of the National Cancer Institute. 1985,74:543–551.
Abrams DB, Orleans CT, Niaura RS, et al.: Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: A combined step care and matching model.Annals of Behavioral Medicine. 1996,18:290–304.
Velicer WF, DiClemente CC: Understanding and intervening with the total population of smokers.Tobacco Control. 1993,2:95–96.
Velicer WF, Prochaska JO: An expert system intervention for smoking cessation.Patient Education and Counseling. 1999,36:119–129.
Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY: Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation.Addictive Behaviors. 2001, 26:583–602.
Velicer WF, Prochaska JO, Fava JL, Laforge RG, Rossi JS: Interactive versus non-interactive interventions and dose-response relationships for stage-matched smoking cessation programs in a managed care setting.Health Psychology. 1999,18:1–8.
Schmid TL, Jeffrey RW, Hellerstedt WL: Direct mail recruitment to house-based smoking and weight control programs: A comparison of strengths.Preventive Medicine. 1989,18:503–517.
Linnan LA, Emmons KM, Klar N, et al.: Challenges to improving the impact of worksite cancer prevention programs: Comparing reach, enrollment, and attrition using active versus recruitment strategies.Annals of Behavioral Medicine. 2002,24:157–166.
Ahluwalia JS, Richter K, Mayo MS, et al.: African American smokers interested and eligible for a smoking cessation clinical trial: Predictors of not returning for randomization.Annals of Epidemiology. 2002,12:206–212.
Fava JL, Velicer WF, Prochaska JO: Characteristics of smokers who refused participation in a longitudinal study. 16th Annual Meeting of the Society of Behavioral Medicine. San Diego, CA: 1995.
Prochaska JO, Velicer WF, DiClemente CC, Guadagnoli E, Rossi JS: Patterns of change: Dynamic typology applied to smoking cessation.Multivariate Behavioral Research. 1991,{26}:83–107.
Velicer WF, Rossi JS, Ruggiero L, Prochaska JO: Minimal interventions appropriate for an entire population of smokers. In Richmond R (ed),Interventions for Smokers: An International Perspective. Baltimore: Williams & Wilkins, 1994, 69–92.
Velicer WF, Prochaska JO, Bellis JM, et al.: An expert system intervention for smoking cessation.Addictive Behaviors. 1993,18:269–290.
Friedman RH: Automated telephone conversations to assess health behavior and deliver behavioral interventions.Journal of Medical Systems. 1998,22:95–102.
Friedman RH, Kazis LE, Jette A, et al.: A telecommunications system for monitoring and counseling patients with hypertension: Impact on medication adherence and blood pressure control. {American Journal of Hypertension}. 1996,9:285–292.
Ramelson HR, Friedman RH, Ockene JK: An automated telephone-based smoking cessation education and counseling system.Patient Education and Counseling. 1999,36:131–144.
Velicer WF, Fava JL, Prochaska JO, et al.: Distribution of smokers by stage in three representative samples.Preventive Medicine. 1995,24:401–411.
Laforge RG, Velicer WF, Richmond R, Owen N: Stage distributions for five health behaviors in the USA and Australia.Preventive Medicine. 1999,28:61–74.
Prochaska JO, Velicer WF, DiClemente CC, Fava JL: Measuring the processes of change: Applications to the cessation of smoking.Journal of Consulting and Clinical Psychology. 1988,56:520–528.
Velicer WF, DiClemente CC, Prochaska JO, Brandenberg N: A decisional balance measure for assessing and predicting smoking status.Journal of Personal and Social Psychology. 1985,48:1279–1289.
Velicer WF, DiClemente CC, Rossi JS, Prochaska JO: Relapse situations and self-efficacy: An integrative model.Addictive Behaviors. 1990,15:271–283.
Fava JL, Velicer WF, Prochaska JO: Applying the Transtheoretical Model to a representative sample of smokers.Addictive Behaviors. 1995,20:189–203.
Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: Toward an integrative model of change.Journal of Consulting and Clinical Psychology. 1983,51:390–395.
Spencer L, Pagell F, Hallion ME, Adams TB: Applying the Transtheoretical Model to tobacco cessation and prevention: A review of the literature.American Journal of Health Promotion.2002,17:7–71.
Cohen J:Statistical Power Analysis for the Behavioral Sciences (Rev. Ed.). New York: Academic, 1977.
Prochaska JO, Velicer WF: The Transtheoretical Model of health behavior change [Invited paper].American Journal of Health Promotion. 1997,12:38–48.
Velicer WF, Prochaska JO, Fava JL, et al.: Using the Transtheoretical Model for population-based approaches to health promotion and disease prevention.Homeostasis in Health and Disease. 2000,40:174–195.
Abrams DB, Herzog TA, Emmons KM, Linnan LA: Stages of change versus addiction: A replication and extension.Nicotine and Tobacco Research. 2000,2:223–229.
Herzog TA, Abrams DB, Emmons KM, Linnan LA, Shadel WG: Do processes of change predict smoking stage movements? A prospective analysis of the Transtheoretical Model.Health Psychology. 1999,18:369–375.
Herzog TA, Abrams DB, Emmons KM, Linnan LA: Predicting increases in readiness to quit smoking: A prospective analysis using the contemplation ladder.Psychology and Health. 2000,15:369–381.
Velicer WF, Sun X, Redding CA, Prochaska JO: Relationship between smoking behavior and smoking cessation across five studies. National Conference on Tobacco or Health. Boston: 2003.
Velicer WF, Norman GJ, Fava JL, Prochaska JO: Testing 40 predictions from the Transtheoretical Model.Addictive Behaviors. 1999,24:455–469.
Prochaska JO, Wright J, Velicer WF: Evaluating theories of behavior change: A hierarchy of criteria applied to the Transtheoretical Model. Manuscript submitted for publication.
Riemsma PR, Pattenden J, Bridle C, et al.: Systematic review of the effectiveness of stage based interventions to promote smoking cessation.British Medical Journal. 2003,326:1175–1177.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant CA71356 from the National Cancer Institute supported this work. Portions of this article were presented at the annual meeting of the Society for Research on Nicotine and Tobacco, Arlington, Virginia, February 2000, and at the International Conference for Behavioral Medicine, Brisbane, Australia, November 2000.
About this article
Cite this article
Velicer, W.F., Keller, S., Friedman, R.H. et al. Comparing participants and nonparticipants recruited for an effectivenes study of nicotine replacement therapy. ann. behav. med. 29, 181–191 (2005). https://doi.org/10.1207/s15324796abm2903_4
Issue Date:
DOI: https://doi.org/10.1207/s15324796abm2903_4