Abstract
Introduction
Cell-based therapy represents a new frontier in the treatment of a wide variety of human diseases traditionally associated with morbidity outcomes, including those involving inflammation, autoimmunity, tissue damage, and cancer. However, the use of mesenchymal stem cells (MSCs) to treat multiple myeloma (MM) bone disease has raised concerns. Specifically, evidence has shown that infused MSCs might support tumor growth and metastasis.
Methods
In this study, we used a standard disseminated MM model in mice to identify the in vivo effects of intravenous MSC infusion. In addition, a series of in vitro co-culture assays were preformed to explore whether Fas/Fas ligand (Fas-L) is involved in the inhibitory effects of MSCs on MM cells.
Results
In the MM mouse model, treatment of MSCs with highly expressed Fas ligand (Fas-Lhigh MSCs) showed remarkable inhibitory effects on MM indenization in terms of extending the mouse survival rate and inhibiting tumor growth, bone resorption in the lumbus and collum femoris, and MM cell metastasis in the lungs and kidneys. In addition, reduced proliferation and increased apoptosis of MM cells was observed when co-cultured with Fas-Lhigh MSCs in vitro. Furthermore, mechanistically, the binding between Fas and Fas-L significantly induced apoptosis in MM cells, as evidenced through an increase in the expression of apoptosis marker and Fas in MM cells. In contrast, Fas-Lnull MSCs promote MM growth.
Conclusions
These data suggest that Fas/Fas-L-induced MM apoptosis plays a crucial role in the MSC-based inhibition of MM growth. Although whether MSCs inhibit or promote cancer growth remains controversial, the levels of Fas-L expression in MSCs determine, at least partially, the effects of MSCs on MM cell growth.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy, with a yearly incidence of 14,000 in the United States, constituting 13% of blood cancers and 1% of all human cancers [1]. MM is unique among most hematological malignancies, with a high capacity to induce osteolytic bone lesions through the suppression of osteoblastogenesis, stimulation of osteoclastogenesis and the subsequent uncoupling of bone resorption and bone formation in areas adjacent to tumor foci in the bone marrow [2]. The occurrence of bone lesions is thus one of the major characteristics of MM patients [3]; the number of osteolytic or focal lesions has been associated with clinical disease progression for patients with MM [4]. More than 80% of MM patients suffer from large osteolytic lesions, and a lack of clinically effective therapeutics leads to the increasing severity of skeletal complications and, indeed, death as a result of lowered resistance to infection (that is, immunodeficiency), hypercalcemia, anemia, and renal failure, among others [5]. It is estimated that MM, with a median overall survival of 3 to 5 years, accounts for approximately 20% of deaths from hematologic malignancy and 1 to 2% of cancer-related deaths overall [6]. Currently, cure is a realistic goal for only a small minority of MM patients. The high morbidity and mortality rates associated with plasma cell malignancy have led to an increased demand for the effective management of this condition.
It is now generally accepted that MM bone disease is a reflection of osteoblast deactivation [3, 7]. The correction of osteoblast function in the bone of MM patients, therefore, has long been a primary target for the design of therapeutics for MM-related bone disease. To this end, bisphosphonates, such as pamidronate, and novel agents, such as lenalidomide, dasatinib, and bortezomib, which inhibit osteoclast activity and/or activate osteoblasts, have been identified for treatment of MM-induced osteoblast deactivation [2, 8]. Increased knowledge of the signaling pathways involved in the regulation of osteoblast formation and differentiation from MSCs might provide a better understanding of the pathophysiological mechanisms involved in MM-induced osteoblast inhibition and permit the development of more potential therapeutic agents against bone damage [2, 8, 9]. In this regard, novel osteoblast-activating agents, such as dickkopf-1-neutralizing antibody [10] and inhibitor of activin A signaling [11], are explored for the clinical treatment of MM bone disease. The combination of traditional cytotoxic and novel agents has led to higher response rates and improved long-term survival compared with treatment with standard doses of chemotherapy alone. Unfortunately, not all patients will respond to established novel agents, and even those who do respond will ultimately relapse or become refractory to currently available regimens. Moreover, large lytic lesions are typically not repaired, even after long-term remission, and relapses often occur in pre-existing lesions [4]. Consequently, additional approaches are urgently needed to achieve systemic bone anabolism and repair large osteolytic lesions.
The use of mesenchymal stem cells (MSCs), also called mesenchymal stem cells, to treat MM bone disease has received considerable attention in the field of stem cell research [12]. However, whether MSC infusion inhibits or promotes cancer growth remains controversial. A number of in vitro studies suggest that MSCs from MM patients possess abnormal genomic, phenotypic, and functional properties, which might contribute to impaired bone formation in this disease by supporting and protecting MM cells from spontaneous and drug-induced apoptosis [9]. Furthermore, recent evidence shows that MSCs, when injected subcutaneously, promote tumor growth and neovascularization in syngeneic mouse models through directly supporting the tumor vasculature and secreting proangiogenic factors [13]. Indeed, the promotion of tumor growth through MSCs has also been observed in various cancer models (reviewed in [14]), suggesting that, at least in some specific conditions, MSCs play important roles in tumor progression. In contrast with evidence supporting the fact that MSCs stimulate tumor growth, other studies have documented the routine suppression of tumor growth through MSCs (also reviewed in [14]). In particular, exogenously administered MSCs effectively promote bone formation and inhibit bone disease and the growth of highly aggressive MM cells in the bone, although the majority of systemically injected MSCs were localized in the lungs or in draining lymph nodes [15]. Furthermore, intrabone-injected MSCs have been demonstrated to act as bystander cells to promote bone formation, inhibit osteolysis, and delay MM growth and regrowth [5, 15]. New insights into the effects of in vivo milieu on MSC functions might explain these contradicting results [Immunofluorescence staining for multiple myeloma cells or MSCs MM cells and MSCs co-cultured on dishes were fixed in 4% formaldehyde for 10 minutes. For fluorescence staining, the samples were treated in 0.5% (V/V) Triton X-100 (Novocastra Laboratories Ltd, Newcastle, UK) for 3 minutes and incubated with Annexin V/7AAD. The other cells were blocked with 10% normal goat serum for 30 minutes at 37°C and incubated overnight at 4°C with anti-Fas (1:100; Chemicon International Inc. Temecula, CA, USA) or anti-Fas-L (1:100; Chemicon International Inc.) antibodies. The samples were incubated with tetramethylrhodamine isothiocynate-conjugated or fluorescein isothiocyanate-conjugated secondary antibodies for 2 hours at 37°C. Imaging was performed using an Axiotech Microscope (Carl Zeiss Co. Ltd, Göttingen, Germany). Data are expressed as mean ± standard deviation for two to four parallel experiments repeated separately. One-way analysis of variance and Fisher’s least-significant difference tests were performed. P <0.05 was considered statistically significant. The experiments were performed in triplicate and repeated twice or more to verify the results.Statistical analysis
Results
Effects of MSC infusion on multiple myeloma model mice
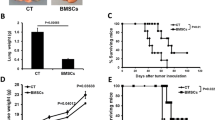
We investigated the 6-week survival rates of mice in each group (for each group, n = 14). The effect of MSC administration on the 6-week survival rates of MM model mice is shown in Figure 1B. The survival rate of MM model mice without MSC or L-PAM treatment (MM group) dramatically decreased until all mice died after 6 weeks of feeding. MM model mice with MSC (MSC group) or L-PAM (L-PAM group) administration showed no difference in the survival rate during the 6-week observation period (P >0.05). Although the survival rate of mice in the MSC group or the L-PAM group was lower than in the Control group (original nude mice without MM cell injection) (P <0.05), the administration of either MSCs or L-PAM achieved prolonged survival compared with the MM group (P <0.05). No animal in the negative control group died (the original nude mice without any treatments) after 6 weeks of feeding.
The number and size of cancroid pearls were compared among the four groups (for each group, n = 14) after 4 weeks of feeding (Figure 1C). No cancroid pearls were identified in the negative control group (Control group), but significant differences in the number and size of the pearls were observed between the MSC and MM groups (P <0.05). Although the number of pearls showed no significant difference, the size of the pearls in the L-PAM group was smaller compared with the MM group (P <0.05). These data indicate that MSC administration inhibits both the number and the size of cancroid pearls in MM model mice, while L-PAM administration inhibits only the size of cancroid pearls.
Furthermore, bone resorption in the lumbus and collum femoris of mice in different groups was examined and statistically analyzed through microcomputed tomography after 4 weeks of feeding (for each group, n = 3) (Figure 1D). The bone density in both the lumbus and collum femoris of animals in the MM group was much lower compared with the BD in the MSC, L-PAM, or Control groups (P < 0.05). Although lower than the Control group (P <0.05), no significant differences were observed between the two treated groups (P >0.05).
In addition, MM cell metastasis was determined and quantified in the lungs and kidneys of MM model mice after 4 weeks of feeding (for each group, n = 5) (Figure 1E). The rate of metastasis in the lung tissue obtained from mice in the MSC group was much lower compared with either the MM or the L-PAM groups (P <0.05). Similarly, no significant difference was observed among the MM, MSC, and L-PAM groups in kidney tissue, although the metastasis rate in the MSC group was lowest (P <0.05).
Effects of MSCs on multiple myeloma cells under co-culture conditions
First, we designed three testing co-culture groups, where the number of MM cells was equal to or five to 10 times greater than that of MSCs. MM cells without MSC co-culture served as the control. In the control group, the MM cell number increased 2.5-fold over the initial cell number at 24 hours after co-culture. When the initial number of MM cells was equal to or five times greater than that of MSCs, the inhibitory effect of MSCs on MM cell increase was apparent. However, no significant MM cell increase was observed when the number of MM cells was 10 times the number of MSCs (Figure 2A).
Relationship between multiple myeloma cells and mesenchymal stem cells under co-culture conditions. (A) Multiplication of multiple myeloma (MM) cells under co-culture with mesenchymal stem cells (MSCs). (B) Western blot analyses of apoptosis markers. Expression levels of cleaved caspase-3 and caspase-8 in MM cells with or without co-culture with MSCs were analyzed through western blotting at 0, 6, 12, and 24 hours (lower table arranged to numerical value from upper data). (C) Apoptotic analysis through fluorescence-activated cell sorting (FACS) (x axis, Annexin V; y axis, 7AAD-positive cells). Apoptosis of MM cells with or without co-culture with MSCs was detected and quantified through FACS at 0, 6, 12, and 24 hours (lower table arranged from upper data). Data presented as mean ± standard deviation (SD) for two parallel experiments. #P <0.05 versus the control group (Cont.). (D) Rate of apoptotic MM cells under co-culture with MSCs. The microscope pictures represent typical reactions of MM cells in fluorescence staining, where MM cells were prestained for carboxyfluorescein diacetate, succinimidyl ester (CFSE; green) and apoptosis markers using Annexin V and 7AAD (red). Lower table arranged to numerical value from upper data. Data presented as mean ± SD for two parallel experiments. #P <0.05 versus the control group (Cont.). DAPI, 4′,6-diamidino-2-phenylindole.
To determine whether the reduction was induced through MM cell apoptosis, caspase-3 and caspase-8 expression in MMs was determined through western blotting. The increased expression of cleaved caspase-3 and caspase-8 was observed in most MM cells after 12 hours of co-culture (Figure 2B). The cleaved caspase-3 and caspase-8 expression in MM cells without co-culture with MSCs was fixed, while the expression of cleaved caspase-3 and caspase-8 in MM cells co-cultured with MSCs was slightly increased. Furthermore, the apoptotic rate of MM cells was examined using fluorescence-activated cell sorting and Annexin V and 7AAD immunofluorescence. We observed that the apoptotic rate of MM cells, determined and quantified through fluorescence-activated cell sorting analysis, was dramatically changed at 12 hours after co-culture with MSCs (P <0.05) (Figure 2C). In the case of immunofluorescence, the number of positive apoptotic markers in MM cells significantly increased after 6 hours of co-culture (P <0.05) (Figure 2D).
Influence of Fas/Fas ligand pathway on multiple myeloma cell apoptosis
Next, we investigated the different conditions of co-culture with MSCs to confirm the necessity of direct contact between MSCs and MM cells for the induction of MM cell apoptosis. Only the direct co-culture group, which shows direct contact between MM cells and MSCs, exhibited a strong inhibitory effect on MM cell growth (P <0.05) (Figure 3A). In addition, the expression of Fas in MM cells and Fas-L in MSCs, determined through western blotting, increased at 12 and 24 hours (P <0.05) (Figure 3B). The data for immunofluorescence staining showed results similar to those of the western blot analysis (Figure 3C).
Fas and Fas ligand analysis in multiple myeloma cells and mesenchymal stem cells under co-culture. (A) Multiplication of multiple myeloma (MM) cells under different co-culture conditions with mesenchymal stem cells ( MSCs) (Cont., MM single culture; C-medium, MM culture with conditioned medium from MSC culture; Trans-well, indirect co-culture between MM and MSC; Co-cul, direct culture between MM and MSC). Each data point represents the mean ± standard deviation (SD) of two parallel experiments. #P <0.05 versus the control group (Cont.). (B) Western blotting analyses of the expression levels of Fas and Fas ligand (Fas-L) in MM cells and MSCs under co-culture condition for 0, 6, 12, and 24 hours. (C), (D) The rates of Fas-positive or Fas-L-positive cells were determined through immunofluorescence staining. (C) MM cells were prestained for carboxyfluorescein diacetate, succinimidyl ester (CFSE; green) and then post-stained for Fas (red). (D) MSCs were stained for Scar-1 (green) and subsequently stained for Fas-L (red). Bar = 300 μm. Numbers of Fas-positive MM cells and Fas-L-positive MSCs are shown. Data presented as the mean ± SD for two parallel experiments. #P <0.05 versus the control group (Cont.). DAPI, 4′,6-diamidino-2-phenylindole.
MSCs from gld mice, which had no Fas-L, and aspirin-treated MSCs, which had high Fas-L, were used to confirm that the enhancement of Fas-L in MSCs increased the apoptosis of MM cells. Aspirin treatment showed the enhanced expression of Fas-L in MSCs (Figure 4A). A reduction in the number of MM cells co-cultured with aspirin-treated MSCs was less than that in the normal co-cultured group (co-cultured with normal MSCs) at 12 hours (P <0.05) (Figure 4B). However, co-culture with the MSCs obtained from gld mice had little influence on MM cell growth. Furthermore, the expression of caspase-3 and caspase-8 in MM cells co-cultured with aspirin-treated MSCs was significantly increased after 12 hours. Under these conditions, the protein expression of cleaved caspase-3 and caspase-8 was reduced after 12 hours. In the case of the gld group, the expression of these proteins remained unchanged (Figure 4C). The rate of apoptotic cell death was further investigated using fluorescence-activated cell sorting (Figure 4D) and immunofluorescence with Annexin V/7AAD (Figure 4E). Aspirin-treated MSCs showed a greater effect on MM cells than normal MSCs (control group) (P <0.05), and MSCs obtained from gld mice showed effects similar to those of normal MSCs.
Effects of Fas ligand levels in mesenchymal stem cells on multiple myeloma cells under co-culture. (A) Comparison of Fas ligand (Fas-L) expression in mesenchymal stem cells (MSCs) after aspirin treatment. (B) Multiplication of multiple myeloma (MM) cells under co-culture condition with MSCs that express different levels of Fas-L. For the graph, the number of MM co-cultured without MSCs (Cont.), with normal MSCs (Co-cul), with MSCs from generalized lymphoproliferative disease mice (Co-cul(gld)) or with MSCs treated with any aspirin (Co-cul(Asp)) were counted after 0, 6, 12 or 24 hours. (C) Apoptotic analysis of MM cells under co-culture condition with MSCs expressing different levels of Fas-L (detected and quantified through fluorescence-activated cell sorting (FACS): x axis, Annexin V; y axis, 7AAD-positive cells). Data presented as mean ± standard deviation (SD) for two parallel experiments. #P <0.05 versus Con. (D) Expression levels of cleaved caspase-3 in MM cells with or without co-cultured MSCs (expressing different levels of Fas-L) at 0, 6, 12, and 24 hours. (E) Rates of apoptotic MM under co-culture with MSCs (expressing different levels of Fas-L). Data presented as mean ± SD for two parallel experiments. #P <0.05 versus the control group (Con.). CFSE, carboxyfluorescein diacetate, succinimidyl ester; DAPI, 4′,6-diamidino-2-phenylindole.
Effects of MSCs with highly expressed Fas ligand on the multiple myeloma model mice
The MSCs obtained from normal, gld or aspirin-treated mice were used to examine the 5-week survival rates of the MM model mice. The survival rate in the MM model mice treated with aspirin was much higher compared with normal MSC-treated mice (P <0.05). Interestingly, the MSCs obtained from the gld mice showed a reduced survival rate (Figure 5A). Furthermore, the number and the size of cancroid pearls were compared among all groups, and both the number and size in the aspirin-treated group were lower compared with the normal group. However, the gld treated group showed a significantly larger cancroid pearl size compared with the MM model mice without MSC treatment (MM group), although the number of pearls in the MSC gld treated group was less than that in the MM group (Figure 5B). Figure 5C showed the distribution of cancroid pearl size in the MM model mice in each group. Moreover, the presence of MSCs and the apoptosis of MM cells in the pearls of the MM model mice were observed through immunofluorescence and TUNEL staining. The presence of MSCs was positive in all MSC injection groups (Figure 5D).
Effect of mesenchymal stem cells with highly activated Fas ligand on multiple myeloma model mice. (A) Five-week survival rates of multiple myeloma (MM) model mice. (B) Comparison of the number (left) and size (right) of cancroid pearls in MM model mice after treatment with mesenchymal stem cells (MSCs) expressing different levels of Fas ligand (Fas-L). Data presented as mean ± standard deviation (SD) for three parallel experiments. *P <0.05 versus MSC group; #P <0.05 versus the control group. Right panel: representative photograph of typical cancroid pearls at the base of the tail (bar = 5 mm). (C) Distribution of cancroid pearl size in the four test groups (n = 14). (D) Apoptosis of MM cells in MM model mice. The pearls in each group were triple-stained for 4′,6-diamidino-2-phenylindole ( blue), Scar-1 (green) and Annexin V/7AAD (red). All groups treated with MSCs had Scar-1-positive cells in the pearls. However, the positive reactions of apoptosis marker in the MSC(gld) group were much lower than those observed in other groups. The MSC(Asp) group had the highest number of reactions among all groups (bar = 5μm). Lower panels stained through the terminal deoxynucleotidyl transferase-mediated UTP nick-end labeling (TUNEL) assay. Positive reactions were observed in both MSC and MSC(Asp) groups. ASP, aspirin; Con, control; gld, generalized lymphoproliferative disease.
Discussion
MM is a malignancy of antibody-secreting plasma cells, where B-cell plasmacytomas stimulate osteoclast activity, and hence bone resorption, resulting in progressive osteolytic lesions [18]. Based on studies concerning the pathogenic role of autoantibodies in MM diseases, recent advances in this field suggest a more central role for B cells in the maintenance of the disease process beyond their roles as precursors for (auto)antibody-producing plasma cells [31]. Particularly, a number of surface molecules and subsequent downstream signaling pathways are involved in the regulation of MM-related bone destroying events, in which bone resorption and formation are no longer balanced as a consequence of the increased activity of osteoclasts, but rather the osteoblast activity is reduced, leading to an uncoupled, or severely imbalanced, bone remodeling process [2]. Clinical data have shown that MM patients with advanced bone lesions might show a reduction of bone formation markers, such as alkaline phosphatase and osteocalcin, together with increased bone resorption markers, such as receptor activator of nuclear factor κB ligand (RANKL) and C-terminal cross-linked telopeptide of type I collagen [32]. Similarly, marked osteoblastopenia and reduced bone formation have also been reported in murine models of MM bone disease [33]. These studies demonstrate that MM cells suppress osteoblast formation and differentiation, and consequently inhibit bone formation.
Recent mounting evidence indicates that MM cells suppress osteoblastogenesis through contact-dependent cell–cell interaction [7, 34] and the production of osteoblast-inactivating factors including Wnt inhibitors, such as dickkopf-1 [10] and secreted frizzled-related protein 2 [35], and cytokines, such as CCL3 (also known as macrophage inflammatory protein-1 alpha) [11], hepatocyte growth factor, and IL-3/6 [18]. Osteolytic lesions in MM are only observed adjacent to intramedullary plasma cell foci or plasmacytomas, supporting the idea that MM cells might secrete factors that promote the activation of osteoclasts and the inactivation of osteoblast function to replace bone loss [36]. More effective approaches to cure MM-related bone disease, in addition to the correction of osteoblast function, should therefore be reflected in therapeutic modalities aimed at inducing MM cell death.
Researchers in the stem cell field are working to translate the knowledge gained from stem cell biology and function into therapeutic breakthroughs and applications. It is well known that osteoblasts originate primarily from MSCs and are responsible for bone matrix synthesis through the secretion of collagen, which forms strands called osteoid [37]. Osteoclast activity is regulated through the expression of cytokines, such as receptor activator of RANKL, which activates osteoclast differentiation, and osteoprotegerin, which acts as a decoy receptor and inhibits RANKL [38]. Based on this knowledge, MSC-based cytotherapy has established a novel concept for the treatment of MM-related bone disease [39]. Recently, Li and colleagues demonstrated that both systemic and intrabone cytotherapeutic strategies were effective and clinically applicable for treating MM-related bone disease [15], where weekly systemic injections of MSCs restrained MM disease progression through the ability of MSCs to traffic to myelomatous bone and survive for a short period of time [5]. Intrabone injections of MSCs, however, not only inhibited tumor growth in the bone with active MM but also effectively promoted bone formation during disease, remission and delayed MM relapse. Although this study provides a proof-of-concept for the use of MSC cytotherapy to treat large, unhealed, osteolytic lesions and for the systemic inhibition of MM bone disease, the mechanisms of action by which MSC cytotherapy stimulates bone formation and inhibits MM-induced bone tumor growth, are, however, only partially understood [15]. Whether MSCs inhibit or promote cancer growth has developed into a controversy reflected in concern over the use of MSCs, which exhibit a propensity to home to tumors. Once resident in the tumor microenvironment, these cells support tumor growth and spread [14], although the ability of cultured MSCs to support long-term growth of primary MM cells is often limited and not reproducible [40, 41]. Therefore, understanding the in vivo milieu in which MSCs either inhibit or enhance MM cell survival and metastasis is crucial both to safely develop MSCs as a therapeutic tool and to advance our understanding of the role of tumor stroma in carcinogenesis [16, 17]. Moreover, we still do not have a general consensus of what defines these MSCs; the polarization of MSCs into a proinflammatory or an immunosuppressive phenotype showing reversed effects on tumor growth has been observed [17, 42]. Similarly, in our present study, Fas-Lhigh MSCs showed significant inhibition, while Fas-Lnull MSCs showed promoted MM growth, suggesting that the levels of Fas-L expression in MSCs determine, at least in part, the effect of MSCs on cancer growth.
Recent findings suggest that the overexpression of growth differentiation factor 15 in bone marrow MSCs occurs widely in patients with MM, and tumor microenvironment-derived growth differentiation factor 15 is a key survival and chemoprotective factor for MM cells, indicating that the behavior of MSCs might be principally determined by the surrounding environment [43]. The two side effects of MSCs on MM cells identified from previous studies are therefore basically acceptable. To further clarify the role of healthy MSCs in MM metastasis and apoptosis, we examined whether outside-infused MSCs would have therapeutic effects on MM cells in mice under co-culture conditions. The data obtained in the present study are clearly inconsistent with some reports, which have indicated that murine and human MSCs promote breast and coronal cancer growth and metastasis [44, 45]. Interestingly, Ma and colleagues showed that human umbilical cord MSCs significantly inhibited the growth of breast cancer cells in vitro and in vivo[46]. Furthermore, the ability of cytotherapy through placenta-derived adherent cells to impact bone remodeling and increase bone formation in nonmyelomatous SCID-rab mice has been demonstrated [15]. Intralesional mesenchymal cell cytotherapy also resulted in inhibiting growth of H929 MM cells and primary MM cells categorized through global gene expression as high risk. Moreover, placenta-derived adherent cells had no effect on the subcutaneous growth of H929 MM cells in SCID mice, and did not confer a growth advantage to MM cells co-cultured with placenta-derived adherent cells or supportive MSCs [47]. Recently, adipose-derived MSCs, engineered to express the pro-apoptotic ligand TRAIL (also known as TNFSF10), killed MM cells and migrated towards MM cells in vitro[48]. These results, together with data in the present study, support the idea that certain phenotypes of MSCs exhibit inhibitory effects on MM cells, such that the anti-myeloma activity of MSCs can be harnessed or enhanced, for example, via gene-modified approaches.
It has been well recognized that MSC therapy potentially offers novel therapeutic modalities that are translatable for clinical treatment of a large variety of pathological conditions or diseases [49]. This development is also true for clinically managing and combating cancer, as MSCs play a central role in the pathogenesis and progression of tumors [5, 50]. MSC administration thus reduces solid tumor growth in mice due to an inhibition of tumor cell proliferation, probably resulting from deep modifications of the tumor angiogenesis, regardless of the tumor model and mode of MSC injection [51]. Clinically, current evidence suggests that cytotherapy markedly increases the proportion of MSCs in bone of MM patients, at least for a short period of time [5, 15]. As deduced from in vitro studies, during this short time, the injected MSCs probably interact with endogenous osteoblast precursors and secrete factors that induce their differentiation into bone-building osteoblasts, while simultaneously directly interactions with osteoclast precursors to secrete factors that attenuate the formation of bone-resorbing osteoclasts [15]. Notably, similar to osteoblasts, MSCs might produce a high level of decorin protein, which inhibits osteoclast formation and promotes osteoblast differentiation [8]. Following the identification of the potential for MSCs to enhance engraftment of hematopoietic stem cells, increase osteoblast activity and suppress osteoclast activity [52], MSCs recruited hematopoietic elements that inhibit inflammatory conditions typically associated with MM growth in bone [15]. Along with recent findings in this field [17, 42], it is speculated that MM progression is restrained, directly and indirectly, through anti-inflammatory factors produced by the injected MSCs or endogenous cells recruited to myelomatous bone after cytotherapy. The findings that MSCs express high levels of anti-inflammatory and antineoplastic factors, such as SERPINF1 and decorin, support this concept [5]. Decorin also attenuates MM cell growth [5]. Although certain soluble factors produced by MSCs might mediate part of their therapeutic activities, cytotherapy at a remote site (subcutaneous) was found to have no effect on MM bone disease or growth [15], suggesting that MSCs must be present in bone marrow to elicit their antimyeloma effects. Indeed, only MSCs injected directly into bone might efficiently induce an antimyeloma environment. Systemically injected MSCs significantly promote bone formation or restrain MM growth because relatively few of those MSCs can transmigrate and traffic to bone [5]. Recent results, however, also suggest that MSCs might be attracted to bone through MM cells or conditions induced through MM or melphalan treatment. More importantly, MSCs might be cleared in various tissues, but exhibit higher survival rates in the implanted bone or lymph nodes and therefore could be detected in these tissues at 2 to 3 days after intravenous or intracardiac injections, respectively [5]. The accumulation of MSCs in lymph nodes, however, might partially explain their immunomodulatory properties. In fact, evidence suggests that intravenously injected MSCs might localize in the lymph nodes of experimental mouse models of autoimmunity [53, 54]. This body of work might also explain the fewer numbers and smaller sizes of cancroid pearls in the neck and root tail of the MSC-treated MM mice in the present study.
Recent studies have revealed that exogenously injected MSCs were not detectable in vivo for long periods of time; the majority of these cells disappeared within 3 to 5 weeks [15, 55]. Clinically, this phenomenon might be advantageous because it limits the duration of the intervention, and these observations support the notion that most of their activities are mediated through the touch-and-go mechanisms of bystander cells, although proof of such evanescence is thus far not well defined [5, 56]. In support of using allogeneic MSCs for MM, Li and colleagues recently demonstrated that intralesionally injected human placenta mesenchymal cells exert similar therapeutic effects in SCID-rab mice [15]. Together, these studies raise an intriguing possibility: if we could understand how MSCs induce MM cell death, then perhaps we could exogenously manipulate MSCs to effectively manage MM and saved a large number of lives. An important, yet unelucidated, question raised by our study is whether a majority of MSCs transmigrate to the myelomatous bone to kill MM cells after intravenous injection, or traffic to lymph nodes to exert inhibitory effects on MM cells via the secretion of anti-inflammatory factors.
The potential role of molecules involved in altered B-cell longevity, particularly those involved in apoptosis (for example, Fas/Fas-L modulators), and those that might alter activation thresholds of B cells in the development of autoimmunity, might contribute to the clinical management of MM [27, 31]. Unfortunately, however, we still know relatively little about this issue. Recently, a number of studies have reported the effects of the Fas/Fas-L pathway on fluoride-induced or melatonin-induced cell apoptosis [57, 58]. In the present study, it was shown that MSCs counterattack MM cells using the same mechanism as observed in other cancer cells; namely, Fas-mediated apoptosis. Fas and Fas-L are co-expressed on primary MSCs that might kill co-cultured MM cells. Co-expression of Fas and Fas-L by MSCs calls into question the functionality of the extrinsic apoptosis pathway in these cells. Although Mazar and colleagues detected Fas expression on MSCs, stimulation of Fas with different concentrations of anti-Fas antibody did not result in any apoptotic response [23]. At this stage, we were not able to identify the exact molecular mechanism of Fas-mediated pathway inactivation in MSCs, but we might narrow this process to events between Fas protein trimerization and caspase-8 activation. As previously demonstrated, caspase-8 deficiency resulted in the inhibition of apoptosis of Jurkat cells, and blocking with Fas Fc protein prevented bone marrow MSC-induced apoptosis in 80% [23]. Other studies have shown that the transformation of the intracellular domain of Fas protein expressed in human MSCs prevents the trimerization of the receptor and blocks the activation of apoptotic pathway activation [59]. MM cells might thus be susceptible to the induction of apoptosis through MSCs.
Briefly, we showed that MSCs act directly on MM cells, inhibiting proliferation. MSC-induced apoptosis in MM cells is evidenced by an increase in the Annexin/7AAD-positive cell population. Most of this effect can be attributed to the Fas/Fas-L pathway. These results confirm and extend previous reports [60, 61]. In our MM model, the activation of both caspase-3 and caspase-8 was observed, suggesting that two main pathways of procaspase activation – the intrinsic mitochondrial pathway and the extrinsic death receptor pathway – are both involved in MSC-induced apoptosis of MM. Having determined the effects of MSCs activated through aspirin (with highly activated Fas-L) in the MM model mice, these MSCs resulted in a more effective clinical outcome compared with MSCs from the gld mice. We thus expected that MSCs with high Fas-L expression would be extremely effective in inhibiting MM growth and metastasis. These results are consistent in principle with those of another study, showing that infused MSCs moved immediately to the tumor site [62]. However, the positive TUNEL reactions within MSCs from the gld mice were much lower than the others, suggesting that MSCs without Fas-L have no capacity to kill MM cells.
Conclusion
Fas-L is expressed on MSCs to induce MM cell apoptosis under co-culture conditions. Furthermore, Fas-L activated through aspirin effectively inhibits the growth and metastasis of MMs in vitro and in vivo. Our results showed the additive or synergistic anti-MM activity of MSCs with highly activated Fas-L, measured on the basis of cell growth, apoptosis, and modest survival improvement of MM-bearing mice, indicating that the levels of Fas-L expression in MSCs determine, at least partially, the effect of MSCs on cancer growth. Our finding has opened up a new way of thinking about the effect of MSCs against MM cells and a new way of moving forward. Further research should aim at improving the trafficking of infused MSCs to myelomatous bone, clarifying and increasing the anti-inflammatory therapeutic potential of these cells during active disease or maintenance therapy compared with the typical administration of drugs, such as L-PAM.
Authors’ information
IA and SL are co-first authors. SS and F-MC are co-last authors.
Abbreviations
- Fas-L:
-
Fas ligand
- gld:
-
Generalized lymphoproliferative disease
- IL:
-
Interleukin
- L-PAM:
-
Lymphocyte Peyer’s patch adhesion molecules
- MM:
-
Multiple myeloma
- MSC:
-
Mesenchymal stromal cell
- PBS:
-
Phosphate-buffered saline
- RANKL:
-
Receptor activator of nuclear factor κB ligand
- TNF:
-
Tumor necrosis factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated UTP nick-end labeling.
References
Bergsagel PL, Kuehl WM: Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005, 23: 6333-6338. 10.1200/JCO.2005.05.021.
Yaccoby S: Advances in the understanding of myeloma bone disease and tumour growth. Br J Haematol. 2010, 149: 311-321. 10.1111/j.1365-2141.2010.08141.x.
Giuliani N, Rizzoli V, Roodman GD: Multiple myeloma bone disease: pathophysiology of osteoblast inhibition. Blood. 2006, 108: 3992-3996. 10.1182/blood-2006-05-026112.
Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Epstein J, van Hemert R, Erdem E, Hoering A, Crowley J, Ferris E, Hollmig K, van Rhee F, Zangari M, Pineda-Roman M, Mohiuddin A, Yaccoby S, Sawyer J, Angtuaco EJ: Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007, 25: 1121-1128. 10.1200/JCO.2006.08.5803.
Li X, Ling W, Khan S, Yaccoby S: Therapeutic effects of intrabone and systemic mesenchymal stem cell cytotherapy on myeloma bone disease and tumor growth. J Bone Miner Res. 2012, 27: 1635-1648. 10.1002/jbmr.1620.
Jemal A, Graubard BI, Devesa SS, Flegal KM: The association of blood lead level and cancer mortality among whites in the United States. Environ Health Perspect. 2002, 110: 325-329. 10.1289/ehp.02110325.
Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, Grano M, Colucci S, Svaldi M, Rizzoli V: Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005, 106: 2472-2483. 10.1182/blood-2004-12-4986.
Li X, Pennisi A, Yaccoby S: Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008, 112: 159-168. 10.1182/blood-2007-11-124164.
Giuliani N, Mangoni M, Rizzoli V: Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: identification of potential therapeutic targets. Exp Hematol. 2009, 37: 879-886. 10.1016/j.exphem.2009.04.004.
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD: Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007, 109: 2106-2111. 10.1182/blood-2006-09-047712.
Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT, Anderson KC, Raje N: A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011, 25: 1174-1181. 10.1038/leu.2011.43.
Gasparetto C: Stem cell transplantation for multiple myeloma. Cancer Control. 2004, 11: 119-129.
Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y: Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011, 17: 579-587.
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F: Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth?. Stem Cells. 2011, 29: 11-19. 10.1002/stem.559.
Li X, Ling W, Pennisi A, Wang Y, Khan S, Heidaran M, Pal A, Zhang X, He S, Zeitlin A, Abbot S, Faleck H, Hariri R, Shaughnessy JD, van Rhee F, Nair B, Barlogie B, Epstein J, Yaccoby S: Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2011, 29: 263-273. 10.1002/stem.572.
Chen L, Wang S, Zhou Y, Wu X, Entin I, Epstein J, Yaccoby S, **ong W, Barlogie B, Shaughnessy JD, Zhan F: Identification of early growth response protein 1 (EGR-1) as a novel target for JUN-induced apoptosis in multiple myeloma. Blood. 2010, 115: 61-70. 10.1182/blood-2009-03-210526.
Waterman RS, Henkle SL, Betancourt AM: Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012, 7: e45590-10.1371/journal.pone.0045590.
Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA: A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006, 24: 986-991. 10.1634/stemcells.2005-0220.
Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, Wallach D, Wiltrout RH, Zörnig M, Lynch DH: The CD95 receptor: apoptosis revisited. Cell. 2007, 129: 447-450. 10.1016/j.cell.2007.04.031.
Strasser A, Jost PJ, Nagata S: The many roles of Fas receptor signalling in the immune system. Immunity. 2009, 30: 180-192. 10.1016/j.immuni.2009.01.001.
Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993, 75: 1169-1178. 10.1016/0092-8674(93)90326-L.
Lettau M, Paulsen M, Kabelitz D, Janssen O: Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr Med Chem. 2008, 15: 1684-1696. 10.2174/092986708784872384.
Mazar J, Thomas M, Bezrukov L, Chanturia A, Pekkurnaz G, Yin S, Kuznetsov SA, Robey PG, Zimmerberg J: Cytotoxicity mediated by the Fas ligand (FasL)-activated apoptotic pathway in stem cells. J Biol Chem. 2009, 284: 22022-22028. 10.1074/jbc.M109.032235.
LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC: Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002, 62: 4996-5000.
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S: Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011, 17: 1594-1601. 10.1038/nm.2542.
Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, Grubbs B, Zhao M, Chen D, Sherry B, Mundy GR: Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 2003, 102: 311-319. 10.1182/blood-2002-12-3905.
Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S: Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012, 10: 544-555. 10.1016/j.stem.2012.03.007.
Musto P, D’Auria F: Melphalan: old and new uses of a still master drug for multiple myeloma. Expert Opin Investig Drugs. 2007, 16: 1467-1487. 10.1517/13543784.16.9.1467.
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD: Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009, 183: 7787-7798. 10.4049/jimmunol.0902318.
Sun LY, Zhou KX, Feng XB, Zhang HY, Ding XQ, ** O, Lu LW, Lau CS, Hou YY, Fan LM: Abnormal surface markers expression on bone marrow CD34+ cells and correlation with disease activity in patients with systemic lupus erythematosus. Clin Rheumatol. 2007, 26: 2073-2079. 10.1007/s10067-007-0621-2.
Dörner T, Lipsky PE: Signalling pathways in B cells: implications for autoimmunity. Curr Top Microbiol Immunol. 2006, 305: 213-240.
Vejlgaard T, Abildgaard N, Jans H, Nielsen JL, Heickendorff L: Abnormal bone turnover in monoclonal gammaopathy of undetermined significance: analyses of type I collagen telopeptide, osteocalcin, bone-specific alkaline phosphatase and propeptides of type I and type III procollagens. Eur J Haematol. 1997, 58: 104-108.
Hjorth-Hansen H, Seifert MF, Börset M, Aarset H, Ostlie A, Sundan A, Waage A: Marked osteoblastopenia and reduced bone formation in a model of multiple myeloma bone disease in severe combined immunodeficiency mice. J Bone Miner Res. 1999, 14: 256-263. 10.1359/jbmr.1999.14.2.256.
Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Barlogie B, Yaccoby S: The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009, 114: 1803-1812. 10.1182/blood-2009-01-201954.
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, Tanaka Y, Shibata H, Hashimoto T, Ozaki S, Kido S, Inoue D, Matsumoto T: Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005, 106: 3160-3165. 10.1182/blood-2004-12-4940.
Bataille R, Manolagas SC, Berenson JR: Pathogenesis and management of bone lesions in multiple myeloma. Hematol Oncol Clin North Am. 1997, 11: 349-361. 10.1016/S0889-8588(05)70435-4.
Gregory CA, Gunn WG, Reyes E, Smolarz AJ, Munoz J, Spees JL, Prockop DJ: How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005, 1049: 97-106. 10.1196/annals.1334.010.
Glass DA, Patel MS, Kassenty G: A new insight into the formation of osteolytic lesions in multiple myeloma. N Engl J Med. 2003, 349: 2479-2480. 10.1056/NEJMp038176.
Fu J, Wang P, Zhang X, Ju S, Li J, Li B, Yu S, Zhang J, Zhang X: Myeloma cells inhibit osteogenic differentiation of mesenchymal stem cells and kill osteoblasts via TRAIL-induced apoptosis. Arch Med Sci. 2010, 6: 496-504.
Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC: The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007, 21: 1007-1034. 10.1016/j.hoc.2007.08.007. vii–viii
Wang X, Zhang Z, Yao C: Survivin is upregulated in myeloma cell lines cocultured with mesenchymal stem cells. Leuk Res. 2010, 34: 1325-1329. 10.1016/j.leukres.2010.05.006.
Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer ZU, Bentrup K, Danka ES, Henkle SL, Scandurro AB: The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA. 2009, 106: 3806-3811. 10.1073/pnas.0900244106.
Corre J, Labat E, Espagnolle N, Hébraud B, Avet-Loiseau H, Roussel M, Huynh A, Gadelorge M, Cordelier P, Klein B, Moreau P, Facon T, Fournié JJ, Attal M, Bourin P: Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012, 72: 1395-1406. 10.1158/0008-5472.CAN-11-0188.
Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K: Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010, 127: 2323-2333. 10.1002/ijc.25440.
Albarenque SM, Zwacka RM, Mohr A: Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011, 7: 163-171. 10.1016/j.scr.2011.05.002.
Ma Y, Hao X, Zhang S, Zhang J: The in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cells. Breast Cancer Res Treat. 2012, 133: 473-485. 10.1007/s10549-011-1774-x.
Caligaris-Cappio F, Bergui L, Gregoretti MG, Gaidano G, Gaboli M, Schena M, Zallone AZ, Marchisio PC: Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991, 77: 2688-2693.
Ciavarella S, Grisendi G, Dominici M, Tucci M, Brunetti O, Dammacco F, Silvestris F: In vitro anti-myeloma activity of TRAIL-expressing adipose-derived mesenchymal stem cells. Br J Haematol. 2012, 157: 586-598. 10.1111/j.1365-2141.2012.09082.x.
Chen FM, Zhao YM, ** Y, Shi S: Prospects for translational regenerative medicine. Biotechnol Adv. 2012, 30: 658-672. 10.1016/j.biotechadv.2011.11.005.
Gomes CM: The dual role of mesenchymal stem cells in tumor progression. Stem Cell Res Ther. 2013, 4: 42-10.1186/scrt189.
Kéramidas M, de Fraipont F, Karageorgis A, Moisan A, Persoons V, Richard MJ, Coll JL, Rome C: The dual effect of mesenchymal stem cells on tumour growth and tumour angiogenesis. Stem Cell Res Ther. 2013, 4: 41-10.1186/scrt195.
Prather WR, Toren A, Meiron M: Placental-derived and expanded mesenchymal stromal cells (PLX-I) to enhance the engraftment of hematopoietic stem cells derived from umbilical cord blood. Expert Opin Biol Ther. 2008, 8: 1241-1250. 10.1517/14712598.8.8.1241.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005, 106: 1755-1761. 10.1182/blood-2005-04-1496.
Parekkadan B, Tilles AW, Yarmush ML: Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008, 26: 1913-1919. 10.1634/stemcells.2007-0790.
Prockop DJ: Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009, 17: 939-946. 10.1038/mt.2009.62.
Uccelli A, Moretta L, Pistoia V: Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008, 8: 726-736. 10.1038/nri2395.
Xu B, Xu Z, **a T, He P, Gao P, He W, Zhang M, Guo L, Niu Q, Wang A: Effects of the Fas/Fas-L pathway on fluoride-induced apoptosis in SH-SY5Y cells. Environ Toxicol. 2011, 26: 86-92. 10.1002/tox.20543.
García-Santos G, Martin V, Rodríguez-Blanco J, Herrera F, Casado-Zapico S, Sánchez-Sánchez AM, Antolín I, Rodríguez C: Fas/Fas ligand regulation mediates cell death in human Ewing’s sarcoma cells treated with melatonin. Br J Cancer. 2012, 106: 1288-1296. 10.1038/bjc.2012.66.
O’Connell J, Houston A, Bennett MW, O’Sullivan GC, Shanahan F: Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med. 2012, 7: 271-274. 2001
Yang X, Yang C, Shao K, Ye X, Meng H, Zhou Y, Qian W: Refractory multiple myeloma treated with homoharringtonine: report of two cases. Ann Hematol. 2007, 86: 919-921. 10.1007/s00277-007-0331-5.
Lou YJ, Qian WB, ** J: Homoharringtonine induces apoptosis and growth arrest in human myeloma cells. Leuk Lymphoma. 2007, 48: 1400-1406. 10.1080/10428190701411466.
Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T: Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006, 203: 1235-1247. 10.1084/jem.20051921.
Acknowledgments
This work was supported through grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449 and R01DE019932), the National Natural Science Foundation of China (31170912), and the Program for New Century Excellent Talents in University (NCET-12-1005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IA, SL, YM and KA were involved in the practical achievement of these experiments. IA, CC, YA and F-MC collected, analyzed and interpreted the data. SS designed the study and provided financial and administrative support. IA, SL, SS and F-MC wrote the manuscript. SL, CC, SS and F-MC revised the manuscript critically for important intellectual content. All authors read and approved the manuscript for publication. Each author participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Ikiru Atsuta, Shiyu Liu, Songtao Shi and Fa-Ming Chen contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Atsuta, I., Liu, S., Miura, Y. et al. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther 4, 111 (2013). https://doi.org/10.1186/scrt322
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/scrt322