Abstract
NLRP inflammasomes are a group of cytosolic multiprotein oligomer pattern recognition receptors (PRRs) involved in the recognition of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) produced by infected cells. They regulate innate immunity by triggering a protective inflammatory response. However, despite their protective role, aberrant NLPR inflammasome activation and gain-of-function mutations in NLRP sensor proteins are involved in occurrence and enhancement of non-communicating autoimmune, auto-inflammatory, and neurodegenerative diseases. In the last few years, significant advances have been achieved in the understanding of the NLRP inflammasome physiological functions and their molecular mechanisms of activation, as well as therapeutics that target NLRP inflammasome activity in inflammatory diseases. Here, we provide the latest research progress on NLRP inflammasomes, including NLRP1, CARD8, NLRP3, NLRP6, NLRP7, NLRP2, NLRP9, NLRP10, and NLRP12 regarding their structural and assembling features, signaling transduction and molecular activation mechanisms. Importantly, we highlight the mechanisms associated with NLRP inflammasome dysregulation involved in numerous human auto-inflammatory, autoimmune, and neurodegenerative diseases. Overall, we summarize the latest discoveries in NLRP biology, their forming inflammasomes, and their role in health and diseases, and provide therapeutic strategies and perspectives for future studies about NLRP inflammasomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The host's innate immune system contributes to recognizing and responding to cellular stress and danger signals [1, 2]. Pattern recognition receptors (PRRs) of the innate immune system mediate recognition of conserved molecular signatures of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [2, 3]. PRRs are usually classified into five main classes by different receptor proteins, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs) and nucleotide-binding domain and leucine-rich repeat receptors (NLRs) [2, 4]. TLRs and CLRs are transmembrane proteins that recognize extracellular PAMPs and DAMPs or within endosomes. The other cited protein receptors, including RLRs, ALRs and NLRs, are thought to detect cytosolic or intracellular PAMPs and DAMPs. Among these receptors, certain NLRs and ALRs can assemble into high-weight oligomeric complexes known as inflammasomes. The term inflammasome was first used by Tschopp and colleagues two decades ago [2, 5].
Inflammasomes are a set of cytoplasmic receptor proteins usually triggered in response to cellular stress associated with infectious agents and physiological aberration. Inflammasomes typically comprise a cytosolic NLR or ALR sensor, an adaptor ASC (apoptosis-associated speck-like protein containing a caspase activation and recruitment domain, CARD), and a cysteine protease caspase-1 [6,7,8]. Based on the different protein components and activation pathways, inflammasomes were traditionally categorized into two main groups: canonical and non-canonical inflammasomes [2, 9, 10]. Canonical inflammasomes were found earlier to form a sensor-ASC-caspase-1 platform for inflammatory caspase-1 activation. Their multiprotein complex formation depends on different cytosolic sensors, mostly from NLR members, ALR members, including AIM2, or the tripartite motif (TRIM) family member, like pyrin [2, 11, 12]. The non-canonical inflammasomes that assemble without dedicated PRRs have similar functions as canonical inflammasomes response to lipopolysaccharide (LPS) and cell endogenous oxidized lipids (oxPAC) by the activation of caspase-11 in mice and caspase-4 and -5 in human [9, 10, 13, 14]. In addition, inflammasomes are widely characterized as protein complexes of activation of inflammatory caspase-1 and a regulated form of cell death called pyroptosis accompanied by DNA fragmentation and rapid plasma membrane permeability [2, 15,16,37] investigating the activation mechanism of inflammasomes in intestinal epithelial cells (IECs) infected with transmissible gastroenteritis virus (TGEV) showed increased levels of pro-inflammatory cytokines (IL-1β, and IL-18) in both IECs and TGEV-infected tissues, with increased transcription and expression of Nlrp1 gene and NLRP1 protein, respectively, and an upgraded activation of caspase-1. Additionally, the TGEV infection-associated high activation of NLRP1 also acts as an interferon-stimulated gene to counteract enterovirus TGEV infection [37]. In other viral infections, such as in Picornaviridae family-related infections and double-stranded RNA Semliki Forest virus infections, NLRP1 has been identified as a sensor that triggers and regulates the protective innate immune response [38, 39].
The role of NLRP1 inflammasome in health and diseases has been investigated. NLRP1 inflammasome has been associated with numerous disease releases. Specifically, certain NLRP1 variants including NLRP1 rs12150220 polymorphism, found in skin inflammatory diseases, such as vitiligo-associated autoimmune diseases, like Addison’s disease, type 1 diabetes, and systemic lupus erythematous, have been associated with a decreased occurrence risk of these diseases [40]. There are many other studies that have reported the involvement of NLRP1 inflammasome in diverse infection-associated immune responses (Fig. 2). How the NLRP1 inflammasome is activated still remains a subject of debating hypotheses. Nevertheless, as we describe bellow, recent reports have raised crucial and concluding facts about the mechanism of NLRP1 inflammasome activation.
Structure of NLRP1 inflammasome
As a member of the NLR family, NLRP1 in humans is the largest member. The structural architecture of human NLRP1 is unique. It comprises a pyrin domain (PYD), followed by a nucleotide-binding domain (NBD), five tandem LRR domains, a ‘function to find’ (FIIND) domain, and a carboxy-terminal caspase activation and recruitment domain (CARD) [57,58,59]. The three asymmetric interaction types of the DD superfamily (Type I, II and III), which have distinct interaction interfaces in each DD superfamily member, are required to assemble complex macromolecular structures. Significantly, we demonstrated that NLRP1CARD co-folds with ASCCARD by these three conserved interfaces and proposed a “Mosaic model” to explain the danger signal transduction amplification in NLRP1 inflammasome [44]. Recent studies also reported the polymerization and assembly mechanism of the C-terminal functional domain of NLRP1 and its analogous CARD8 [60, 61]. In the UPA-CARD filament structure of NLRP1, the CARD domain is located in the core of the filament, and the UPA subdomain, located outside the core filament, is disordered because of a long flexible linker between UPA and CARD. The UPA subdomain enhances the polymerization of the CARD domain, facilitating the filamentous complex formation. Another NLRP1 CARD filament structure comprises CARD dimers outside the core CARD filament that differs from other CARD filaments [60, 61]. These higher-order filamentous complexes have deep biological implications in inflammasome activation and signal transduction amplification.
Physiological activation of NLRP1 inflammasome by PAMPs
Lately, murine-based studies provided more decisive insight into the activation mechanism of NLRP1 (Fig. 4). The Lethal Factor (LF) from Bacillus anthracis of PAMPs is the best-characterized NLRP1 activator that activates a subset of murine NLRP1B (mNLRP1B) and rat NLRP1 (rNLRP1B) proteins [62, 63]. Studies on the mNLRP1B inflammasome along with the identification of the natural physiological stimuli LF of Bacillus anthracis represent a major breakthrough in our understanding of NLRP1 inflammasome activation mechanism [31]. The specific activator anthrax lethal toxin consists of a protective antigen and a lethal factor that could trigger NLRP1B inflammasome assembly to activate caspase-1 and secret IL-1β, but not human NLRP1 inflammasome. In this regard, the FIIND domain of mNLRP1B first auto-proteolysis to produce two non-covalently associated N-terminal and C-terminal fragments. LF mediates the cleavage of the mNLRP1B amino-terminal domain to expose a destabilizing neo-N terminus [64, 65]. Subsequent N-end rule E3 ligase UBR2 recognizes and ubiquitinates the destabilizing N-degron, which is rapidly degraded by proteasome-mediated degradation machinery [66]. The active C-terminal fragment is not degraded because of the break within the FIIND domain but rather is liberated to recruit the pro-inflammatory caspase-1 protease. Activated caspase-1 subsequently induces pyroptotic cell death and inflammatory cytokine releases, such as IL-1β and IL-18 [67]. The unifying mechanism of proteasome-dependent NLRP1 inflammasome activation has been named “functional degradation” [50, 67]. Recently, Sandstrom and co-workers found that IpaH7.8 of Shigella flexneri leads to NLRP1B-dependent IL-1β production in the reconstituted NLRP1B inflammasome system [67]. In mice, Shigella infection induces cytokine production and causes macrophage pyroptosis by IpaH7.8-mediated NLRP1B activation. These data are consistent with the “functional degradation” model of NLRP1 inflammasome activation and suggest that the N-terminal fragment of NLRP1 might act as a kind of tripwire in the detection of pathogens. Specifically, the N-terminus of NLRP1 might serve as a decoy and is sensed by pathogenic protease activity that could destroy mammalian NLR receptors to evade detection by the innate immune system. But the degradation of the N-terminus activates NLRP1 inflammasome and causes an effector-triggered immunity to achieve the function of efficient detection and clearance for foreign pathogens.
The activation trigger and activation mechanisms of human NLRP1 and mouse NLRP1 are highly divergent. The molecular activation mechanism of human NLRP1 remains enigmatic because no physiological activators of human NLRP1 have been found for a long time. Recent studies have started to describe its danger activation signals and illuminate its functional relevance. First, it has been shown that an enteroviral 3C protease of human rhinovirus (HRV) was identified as a physiological activator of human NLRP1 [38, 68]. The authors discovered that 3C protease directly cleaves the human NLRP1 between Q130 and G131 in the N-terminal fragment. 3C protease cleavage of NLRP1 leads to the degradation of N-terminal fragment and liberation of C-terminal UPA-CARD. As mentioned above, the C-terminal functional domain will result in the assembly of active inflammasome and subsequent cytokine release, which is in line with the “functional degradation” model. The 3C protease was identified as the first pathogen-associated activator of human NLRP1 in human primary airway epithelial cells. Second, a recent manuscript reported that SARS-CoV-2 3CL protease NSP5 could trigger an NLRP1-mediated inflammasome response to decrease the production of infectious viral particles [69]. Mechanistically, human NLRP1 is cleaved at the Q333 site by SARS-CoV-2 3CL protease and activated by the “functional degradation” model, similar to what has been found for enteroviral 3C protease. Notably, SARS-CoV-2 3CL protease NSP5 acts as a virulence factor against GSDMD-dependent pyroptosis, but it promotes cell death by caspase-3/GSDME-mediated pyroptosis pathway upon SARS-CoV-2 infection. Third, the other extraordinarily different activator, long double-stranded RNA (dsRNA), is discovered to activate NLRP1 inflammasome in keratinocytes [39]. Semliki Forrest virus (SFV), a positive-sense single-stranded RNA virus, was found by testing different types of viruses. The autoproteolytic and proteasome activity were necessary for dsRNA-induced human NLRP1 activation, suggesting the N-terminal “functional” proteasomal degradation is involved in this process. Using recombinant proteins, human NLRP1, but not murine NLRP1B could directly interact with dsRNA by its NACHT-LRR domains with high affinity. DsRNA binding to NLRP1 enhanced ATPase activity to achieve a conformational switch that releases an active carboxy-terminal fragment. Fourth, Yang and co-workers described a proteasome-independent activation mechanism of NLRP1 inflammasome in 2022 [77]. Reasonably, reductive stress and peptide accumulation could increase the activation level of NLRP1-mediated inflammasome more than either signal alone.
Furthermore, these radical-trap** antioxidants synergize with VbP, thus initiating more inflammatory cytokine secretion and pyroptotic cell death. Because these radical-trap** antioxidants also prevent an iron-dependent form of cell death named ferroptosis, a subsequent study reported that ferroptosis is linked with NLRP1 inflammasome in a model of oxidative stress [78]. The results of cytological experiments showed that the extent of NLRP1 inflammasome is reduced or increased with changes in ferroptosis activity, and the interactive relationship of NLRP1 inflammasome and ferroptosis is demonstrated under oxidative stress. Consistent with this mechanism above, O3, one of the most toxic pollutants, could be sensed by NLRP1 inflammasome in human keratinocytes [79]. Oxidative stress event caused by O3 exposure induces UBR2-mediated ubiquitination and proteasomal degradation of NLRP1, resulting in NLRP1 inflammasome assemble and inflammatory cytokines release.
Finally, the constitutive expression of NLRP1 in keratinocytes perhaps indicates that NLRP1 is engaged in response to ultraviolet B (UVB) exposure [80, 81]. UVB and toxin-induced ribotoxic stress response (RSR) were recently discovered to induce human NLRP1 inflammasome activation by direct phosphorylation [82, 83]. Mechanistically, low-irradiance UVB results in the activation of RSR kinase ZAKα and its downstream effector p38 to directly phosphorylate the disordered linker region between PYD and NACHT domains in human NLRP1. Hyperphosphorylation of disordered region by ZAKα and p38 induce inflammasome assembly and NLRP1-driven pyroptosis. Importantly, they found that stimulation of ZAKα and p38 is sufficient to induce NLRP1 activation in a DPP8/9-independent manner. They also found that NLRP1 is a versatile receptor that could integrate diverse stress signals by its different regions. Nevertheless, these findings suggest that NLRP1 is a complex receptor that responds to pathogens infection but also engages in maintaining host homeostasis.
DPP8/9 inhibitors: the common activator for human and mouse NLRP1 inflammasome
VbP is a small molecule inhibitor of fibroblast activating protein (FAP) and dipeptidyl peptidase family, including DPP4, DPP7, DPP8 and DPP9. VbP is originally used to induce cytokine production and stimulate anti-cancer immune responses in mice [84, 85]. In 2018, VbP was found to induce NLRP1B-mediated pyroptotic cell death in mouse macrophages [86]. Consistent with the conserved nature of DPP8/9, Zhong et al. discovered that human NLRP1 mediates VbP-induced inflammasome activation in keratinocytes [87]. Since this report, VbP has been shown to activate functional rodent alleles and human NLRP1, thus becoming the first known universal NLRP1 activator.
Subsequent studies investigated the VbP-induced activation mechanism of NLRP1 inflammasome. DPP9 was demonstrated to bind to NLRP1 under steady-state conditions using immunoprecipitation assays, in which the interaction was abolished in VbP-treated cells [87]. The interaction of DPP9 with hNLRP1 and mNLRP1 is common, as the DPP9-binding domains in NLRP1 are the most homologous FIIND domains. For the inhibitory effect on NLRP1 inflammasome, the binding of DPP9 and its catalytic activity are a prerequisite. Recently, two back-to-back articles report cryo-EM structures of NLRP1-DPP9 complex, respectively [88, 89]. Interestingly, the NLRP1-DPP9 complex is a ternary complex that consists of DPP9, full-length NLRP1 and the UPA-CARD of NLRP1. In the tripartite complex, the FIIND and UPA subdomain of NLRP1 are visible in the cryo-EM map density, while other domains are not discernible because of the flexible linker. For the first FIIND domain of full-length NLRP1, this structure revealed that the first β-strand of the UPA subdomain inserts into the ZU5 fold, like the autoinhibited FIIND domain. Surprisingly, DPP9 formed a homodimer that captures the second UPA-CARD of NLRP1 to suppress UPA-CARD from self-oligomerization during homeostatic protein turnover. For the second NLRP1 molecule, only the UPA subdomain was discernible, and the disordered N-terminal region (S1212-N1224) folds into the DPP9’s active-site tunnel, similar to the substrate-bound DPP9 structure (Fig. 3b). However, the N-terminus was not cleaved by recombinant DPP9 due to the difference in the binding pose with how substrates bind. Therefore, the results suggest that NLRP1 could be sequestered by DPP9 rather than act as a bona-fide substrate.
Next, the structure of NLRP1-DPP9 in the presence of VbP was solved to reveal that VbP forms a covalent bond with catalytic S730 residue of DPP9 and displaces the interaction between DPP9 and UPA of NLRP1 from the substrate tunnel, which is consistent with the reports that VbP diminishes the interaction of NLRP1 and DPP9 [87]. Another notable aspect of this complex is that the UPA-UPA interaction of two NLRP1 molecules is important in both NLRP1 repression and NLRP1 activation. Mutations on the interface led to the autoactivation of NLRP1 and abolished the UPA-mediated oligomerization, which aligns with UPA-mediated CARD filament formation [60, 61]. Altogether, these data provide important insights into how DPP8/9 negatively regulates NLRP1 inflammasome activation.
NLRP1 inflammasome regulation and dysfunction
Regulation of NLRP1 inflammasome activation
As previously described, activation of NLRP1 inflammasome is associated with host immune response against infections. In contrary, inappropriate and/or excessive activation of NLRP1 inflammasome are associated with severe pathologies. Thus, NLRP1 inflammasome activation should be regulated to prevent such pathologies.
The first downregulation of NLRP1-inflammasome activation occurs at the NLRP1 CARD and FIIND domains. In fact, as previously described, to activate NLRP1-inflammasome, human NLRP1 or murine NLRP1b undergo an auto-proteolysis within the FIIND domain, releasing N- and C-terminal fragments, remaining in an auto-inhibited state, where they are ready to recruit NLRP1 cognate to process the activation of NLRP1 inflammasome, IL-1β release, and macrophage pyroptosis. Abolishing FIIND autolytic proteolysis processing activity blocks and downregulates NLRP1 inflammasome activation [45, 90].
Furthermore, in resting macrophages, as well as after infection clearance, NLRP1 inflammasome should be shut down, and its activation, downregulated. Bcl-2 and Bcl-XL proteins, initially known to regulate apoptosis, were found to interact with NLRP1 protein (but not with other NLRP proteins) and prevent activation of NLRP1 inflammasome [48]. Specifically, Bcl-2 and Bcl-XL proteins would form a complex (Bcl-2/XL) that recognizes and binds to NLRP1, which suppresses the NLRP1-mediated activation of caspase-1 and subsequently prevents production of IL-1β [91]. The negative regulation of Bcl-2 and Bcl-XL was also demonstrated in immune escape mechanisms by certain viruses (including Vaccina virus) that produce viral proteins (such as F1L protein) structurally similar to BcL-2 and BcL-XL, which interact with NLRP1 and downregulate NLRP1 inflammasome activation, which is however favorable for viral replication and spread [48, 92]. Specifically, like BcL2/XL protein complex, F1L protein of Vaccina virus binds to the ATP-binding site on NLRP1, impeding ATP recruitment by NLRP1 and blocking activation of NRLP1 inflammasome. In vitro assay with Vaccinia virus mutants that lack F1L demonstrates a significant production of IL-1β production in human THP-1 cells, which confirms the negative NLRP1 inflammasome regulation [92]. Besides that, the intracellular ORF63 protein was also found to downregulate NLPR1 inflammasome activation and impede production of IL-1β, through binding with NLRP1 and NLRP3, in THP-1 macrophages that were sensed and stimulated by MDP (muramyl dipeptide). In presence of a cognate NLRP1 inflammasome stimulus, downregulation by Bcl-2/XL complex protein and ORF63 is by-passed to prevent their binding to NLRP1 and allow activation of NLRP1 inflammasome [48]. However, the mechanism of blocking the homeostatic effect of Bcl-2, Bcl-XL, and ORF63 remains unclear.
NLRP1 inflammasome dysregulation and associated diseases
Indeed, when the regulation of NLRP1 inflammasome activation is disrupted, it can lead to the occurrence of disease. Many studies have found that NLRP1 can produce pro-inflammatory cytokines after activation, mediate inappropriate inflammation and participate in a variety of physiological immune responses, suggesting that NLRP1 inflammasome contributes in many disease development processes (Fig. 2 and Table 1). Specifically, this aberrant activation of NLRP1 inflammasome have been associated with mutations found in NLRP1, which in turn have mainly been associated with occurrence of diseases, including severe chronic obstructive pulmonary disease (COPD) [93], systemic lupus erythematosus [94], type 1 diabetes [95], vitiligo-associated autoimmune diseases [20, 55, 96, 97], inflammatory bowel disease [98], arthritis, dyskeratosis, psoriasis, multiple self-healing palmoplantar carcinomas (MSPCs) and familial keratosis lichenoides chronica (FKLC) [43, 99] (Table 1).
When the rare gain-of-function mutations on the PYD or LRR domain of NLRP1 were described in 2016, the NLRP1 stepped into the spotlight in skin-related inflammatory pathologies [43]. Notably, MSPC patients carry inherited mutations within the N-terminal PYD domain (A54T, A66V and M77T), and patients with FKLC display an in-frame deletion (F787-R843) in the LRR domain. As expected, these mutations were confirmed to perturb this auto-inhibitory activity because MSPC mutations disrupt PYD folding and FKLC deletion may weaken NLRP1 auto-inhibitory function. Thus, the PYD and LRR domains are thought to play an auto-inhibitory role in NLRP1.
Furthermore, L155H and M1184V are two polymorphisms of NLRP1 that will increase the risk for vitiligo disease. Mechanistically, M1184V causes a significantly increased processing of pro-IL-1β by caspase-1 in the reconstituted HEK293T system, suggesting a potential disease-associated molecular mechanism [45]. The T755N mutation of NLRP1, located within the linker between the NACHT and LRR domain, resulted in a syndromic named juvenile-onset recurrent respiratory papillomatosis (JRRP) [104]. Auto-inflammation with arthritis and dyskeratosis (AIADK) patients who displayed skin lesions, polyarthritis and periodic fever with increased caspase-1 and IL-18, carry R726W and P1214R mutations [113]. The P1214R mutation is close to the cleavage site of the NLRP1 FIIND domain and abolishes NLRP1-DPP9 interaction to result in the auto-activation of NLRP1 and subsequent inflammasome signaling [87]. Furthermore, gain-of-function mutations in lung NLRP1 have been associated with occurrence of a rare upper airway inflammatory disease caused by the human papilloma virus [104]. However, the molecular mechanisms that most mutations of NLRP1 lead to these auto-inflammatory diseases are required to investigate the detailed role of NLRP1 further.
CARD8 inflammasome: an nlrp1 analogous inflammasome
CARD8 inflammasomes and its role in health and innate immunity
Recent breakthroughs and in-depth studies have demonstrated the existence of an NLRP1 inflammasome-like hetero-multimeric complex protein forming an inflammasome, also known as NLRP1 analogous inflammasome or CARD8 inflammasome. The discovery of the CARD8 inflammasome occurred during characterization of the pyroptosis-inducing activity of the non-selective dipeptidyl-peptidase (DPP)-inhibitor Val-boroPro (VbP, Talabostat) and its associated compounds. Indeed, while it is noteworthy that VbP triggers caspase-1-associated pyroptosis [114, 115] via activation of human NLRP1 [86] or mouse NLRP1B [87], it has been also found that upon VbP treatment in human keratinocytes, CARD8 could trigger pyroptosis. Specifically, after inhibition of DPPs using VbP in human myeloid leukemia cells, NLRP1 was found intact and inactive while a CD4+ and CD8+ T-cell death process, characterized as pyroptosis, occurred. Finally, it was demonstrated that this pyroptosis depends on the CARD8-caspase-1-GSDMD-associated pathway and only occurred in resting but not in active T-cells [116]. Of interest, unlike NLRP1 inflammasome, which is present in both human and murine systems (to a lesser extent), CARD8 inflammasome has only been identified in humans and not in murine systems [117, 118]. CARD8 inflammasome uses the FIIND domain and its CARD domain as sensors to directly interact with and activate caspase-1 [61, 116], and has been mainly evidenced from HIV-1 infection-associated inflammatory response [119].
Recent breakthroughs in CARD8 inflammasome
CARD8 is the only other human inflammasome mediator highly similar to NLRP1, with highly similar domain organization that includes the FIIND domain with the self-cleavage site and carboxyterminal CARD domain (Figs. 1 and 3). The structured N-terminal PYD, NOD and LRR domains of NLRP1 are replaced by a disordered N-terminal region in CARD8. The auto-proteolytic activity of FIIND domain of CARD8 results in a non-covalent association between N- and C-termini of CARD8, similar to what has been found in NLRP1 inflammasome. The N-terminal fragment of CARD8 can be degraded by a functional degradation model, and the bioactive C-terminal UPA-CARD of CARD8 is used to form an inflammasome, directly interacting with the CARD of proCaspase-1 for inflammasome activation. For CARD8 and NLRP1, the FIIND domain associates with DPP8/9 to sequester the bioactive component in a ternary complex for restricting the spontaneous inflammasome activation [88, 89, 120] (Fig. 3c). Furthermore, the CARD8 T60 variant is found that it directly interacts with NLRP1 to act as a negative regulator to control the NLRP1 inflammasome activation level [121].
CARD8 and NLRP1 are the tripwire sensors that are activated by pathogen-encoded activities. The human immunodeficiency virus 1 (HIV-1) protease could trigger CARD8 inflammasome assembly to activate caspase-1 and secret IL-1β that resembles NLRP1 [119]. In this regard, HIV protease cleaves N-terminal fragment between phenylalanine (F) 59 and F60 to expose a destabilizing N-degron that can be ubiquitylated for degradation machinery. Subsequent proteasome-mediated degradation of the N-terminal protein fragment removes the self-inhibition function of CARD8 and liberates the C-terminal UPA-CARD to assemble the CARD8 inflammasome. Several studies have reported that the usage of non-nucleoside reverse transcriptase inhibitors (NNRTIs) could lead to protease activity of HIV to kill infected cells, which is due to the activation of CARD8 inflammasome [122].
Furthermore, under DPP9 inhibitors treatment conditions, CARD8 inflammasome will reduce the activation threshold to effectively clear the HIV-infected cells [123]. Additionally, CARD8 could act as an important immune sensor of infection by positive-sense RNA viruses, including Coronaviridae, Picornaviridae and Retroviridae [124]. For the detailed mechanism, the 3CL protease encoded by these RNA viruses could cleave the unstructured N-terminal region of CARD8, leading to the release of C-terminal CARD-containing fragment that is sufficient for inflammasome assembly. DPP8/9 inhibitors, VbP, accelerate the degradation of CARD8 to destabilize the repressive ternary complex for CARD8 inflammasome activation [120]. Structural and biochemical studies of the ternary complex revealed that CARD8 and NLRP1 directly interact with DPP8/9, but only the neo-N terminus of NLRP1 binds to the DPP8/9 active site. VbP could disrupt this interaction between NLRP1 and DPP8/9, but not CARD8 and DPP8/9, to activate human NLRP1 and CARD8 inflammasome [88, 89, 120, 125]. CARD8 inflammasome was found to be required for VbP-induced pyroptosis in human macrophages and resting lymphocytes expressing more CARD8 than NLRP1, and NLRP1 inflammasome is indispensable for VbP-induced cell death in skin and airway epithelial cells with high expression of NLRP1 [116,117,118].
The danger-related signals detected by CARD8 inflammasome have not yet been fully established. A recent study reported that the protein fold disruption could induce proteasome-mediated degradation and cause cytosolic peptide accumulation, destabilizing the CARD8-DPP8/9 ternary complexes to activate the CARD8 inflammasome [75]. On the other hand, the M24B aminopeptidases have been identified to regulate the CARD8 inflammasome activation recently [126]. The M24B aminopeptidases prolidase (PEPD) and X-prolyl aminopeptidase 1 (XPNPEP1) could catabolize peptides named Xaa-Pros that contain a P2 proline (Xaa is any amino acid). When PEPD/XPNPEP1 is inhibited, the accumulation of Xaa-Pros will weakly inhibit DPP8/9 activity, selectively activating the CARD8 inflammasome but not the related NLRP1 inflammasome. The other danger signal that could activate CARD8 inflammasome is reductive stress. A recent study characterized that a radical-trap** antioxidant, JSH-23, induces reductive stress and accelerates the N-terminal degradation of CARD8 [77]. The radical-trap** antioxidant works synergistically with VbP to induce more pyroptotic cell death and inflammatory cytokine secretion. In recent years, many advances in the CARD8 field propelled our understanding of its function, but future studies are needed to determine the more detailed molecular mode of action of CARD8.
NLRP3 inflammasome
NLRP3 inflammasome and its role in health and innate immunity
Primary localized in the microglia, NLRP3 is another member from the NLRP family, discovered to be associated with and form inflammasome after NLRP1 inflammasome, but is the first to be extensively described and well-characterized amongst the canonical NLRP inflammasomes. Described for the first time in human brain, NLRP3 inflammasome consists of NLRP3, ASC, and pro-caspase-1; its detailed structure is described herein. Unlike NLRP1 inflammasome, NLRP3 inflammasome senses a wider variety of activator/stimuli (including TLR agonists [LPS, nigericin, monosodium urate crystals, and ATP], pathogens [fungi, bacteria, and viruses], pro-inflammatory cytokines [tumor necrosis factor, TNF], intracellular components [reactive oxygen species, ion flux, lysosomal disruption-, mitochondrial dysfunction-, metabolic changes and trans-Golgi catabolism-associated components]) [3, 23, 127, 128]. In microglia, NLRP3 inflammasome is activated by proteins such as misfolded or aggregated amyloid-β, α-synuclein and prion protein or superoxide dismutase [129], and members of the complement pathway, and induces production of IL-1β and IL-18 [130]. The NLRP3 inflammasome has been found to be involved in almost all aspects of health and diseases (Fig. 2 and Table 1). For instance, in the majority of health threats, including auto-inflammatory, metabolic, neurodegenerative, and some infectious diseases [128], expression of NLRP3 has been found to be increased alongside with high levels of IL-1β, and IL-18 production, which has attracted an impressive interest for research. Thus, this has been the main reason that justifies its deep and well characterization.
Structural and functional organization of NLRP3 inflammasome
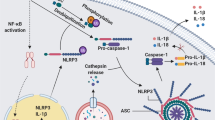
The NLRP3 inflammasome is a multiprotein complex mediating the secretion of proinflammatory cytokines IL-1β and IL-18 and inducing inflammatory cell death (pyroptosis). Also known as NALP3, cryopyrin, PYPAF1, CIAS1, and CLR1.1, the NLRP3 inflammasome has been so far the first extensively and best-characterized canonical inflammasome of the NLRP inflammasomes. NLRP3 inflammasome has been named after its main protein (the NLRP3), which acts like the sensor of the inflammasome, and complexed with two other proteins, including the apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) serving as the adaptor, and the enzyme pro-caspase-1 serving as the effector. Specifically, each component of the NLRP3 inflammasome contains active domains playing crucial roles in the activation and functions of NLRP3 inflammasome. The structure of NLRP3 protein contains 3 active domains, including a central nucleotide-binding and oligomerization (NACHT, aka NOD) domain, flanked by an N-terminal pyrin domain (PYD) domain and a C-terminal leucine-rich repeat (LRR) domain. The central NACHT domain mediates nucleic acid ligation and promotes protein oligomerization; the N-terminal PYD domain is involved in the association of NLRP3 and caspase-1 through interaction with ACS protein; the C-terminal LRR domain is involved in recognition and binding of the inflammasome to putative ligands, including PAMPs and DAMPs, respectively, thus facilitates activation of the NLRP3 inflammasome. The adaptor ASC protein of the NLRP3 inflammasome consists of two domains, including an N-terminal PYD and a C-terminal caspase recruitment (CARD) domain, from which the name PYCARD was attributed. It promotes the binding of NLRP3 (through homotypic PYD-PYD interaction) and pro-caspase-1 (through homotypic CARD-CARD interaction). The enzyme pro-caspase-1 is also a two-domain protein, which consists of a CARD and a caspase domain, containing two sub-units p20 and p10 that act as a catalytic domain. The p20 is the central large catalytic subunit while p10 is the C-terminal small catalytic subunit [106, 128, 131, 132] (Fig. 1).
Upon stimulation, a cascade of protein–protein interactions occurs and ends up in the formation of the active NLRP3 inflammasome complex (Fig. 5). Specifically, the upstream signals activating the NLRP3 inflammasome induce oligomerization, a typically thought conformational changes of NLRP3 protein. The oligomerized NLRP3 in turn uses its N-terminal PYD domain to recruit the adaptor ASC protein through a homotypic PYD-PYD interaction with the N-terminal PYD domain of ASC and nucleates helical ASC filament formation. Subsequently, the adaptor ASC protein, in the form of a complex of fused multiple ASC filaments (aka ASC speck) [56, 128, 129], uses its C-terminal CARD domain to recruit the enzyme pro-caspase-1 through a homotypic CARD-CARD interaction with the N-terminal CARD domain of the effector. Interestingly, extensive studies on the structural organization and components of NLRP3 inflammasome have revealed that NIMA-related kinase 7 (NEK7), a serine-threonine kinase involved in mitosis, also interacts with NLRP3 and contributes to NLRP3 inflammasome activity, thus is an entire component of the NLRP3 inflammasome [133,134,135]. Specifically, from the upstream activation steps, NEK7 binds to and oligomerizes together with NLRP3. This oligomerized complex is essential in recruiting the adaptor ASC protein, but especially favoring ASC speck formation, inducing nucleates helical ASC filament formation, and caspase-1 activation [134, 135]. The NLRP3–ASC–Pro-Capase-1 multiprotein oligomeric complex is the active form of NLRP3 inflammasome, which mediates the proximity-related self-cleavage of pro-caspase-1 to generate the active caspase-1. Then, the catalytic active subunit p20/10 is released from the self-cleavage in the form of heterotetramer, which accomplishes the enzymatic activity of caspase-1, including activation of specific pro-inflammatory cytokines, including pro-IL-18 and pro-IL-1β into IL-18 and IL-1β, their biologically active mature form [136, 137] (Fig. 5 [132]). Upon IL-18 and IL-1β cytokine released, the active subunit p20/10 is degraded, as it is instable in cell cytosolic environment [106, 128, 131, 132, 136].
The mechanisms of human NLRP3 inflammasome activation and regulation. The activation of NLRP3 inflammasome occurs either through a canonical two-step pathway or a non-canonical pathway, and a direct or alternative pathway. The canonical activation pathway involved 2 steps: a priming (signal 1, left panel) and an activation (signal 2, second panel from left) steps. Priming step is induced by NLRP3 signals, including LPS and TNF, IL-1b, IFNs, lipopolysaccharide (LPS), and sphingosine-1 phosphate (S1P), activate NF-κB that; in turn upregulates the transcription of Nlrp3 gene and other genes (ASC and pro-caspase1) involved in NLRP inflammasome, by interacting with and triggering their receptors. Once transcribed, NLRP3 is pre-activated by interacting with NEK7, forming a complex that will be activated into hetero-complex inflammasome. The canonical activation of NLRP3 inflammasome is induced by signal 2 including PAMPs (nigericin, viral RNA, and MDP) and DAMPs (extracellular ATP, mtDNA, and mtROS) and particulates. The molecular mechanisms behind the polymerization and the activation of NLRP3 inflammasome include activation of several signaling events, including induction of K+ efflux, Ca2+ flux, Cl–efflux, lysosomal disruption, mtROS production, and release of oxidized mtDNA in the cytosolic compartment. Thus, formation of NLRP3 inflammasome includes oligomerization of NLRP3-NEK7, recruitment of ASC, and Casp1. auto-proteolysis of proteolytic cleavage of Casp1 releases p10/p20 active enzyme, which digest Pro-IL-1β and Pro-IL-18 into IL-1β and IL-18 cytokines to promote proinflammatory responses. The subunit p10/p20 of Casp1 also digests GSDMD releasing GSDMD-N that form cell membrane pore to result in pyroptosis of the cell. Non-canonical activation of NLRP3 inflammasome (third panel from left) occurs without priming, as Casp4 is already present in the cytoplasm, and is induced by gram-negative bacteria that release LPS into the cell cytosol. Released LPS activates Casp11 in human (and Casp4/5 in mouse), which cleaves GSDMD complex releasing GSDMD-N that forms gasdermin pores and induces pyroptosis. The gasdermin pore formed constitutes a channel for K+ efflux, which activates the NLRP3 inflammasome, and consequently activate Casp1 and IL-1β and IL-18. The alternative pathway (right panel) activation is induced by TLR4 agonists that activates the TLR4-TRIF-RIPK1-FADD-Casp8 signaling pathway. Consequently, Casp8 activates the NLRP3 inflammasome. Note that, there is no need of K+ efflux, ASC speck formation, to activate inflammasome, and there is no pyroptosis
Molecular mechanism of NLRP3 inflammasome activation
The NLRP3 inflammasome is highly expressed in human cells that contribute to the immune defense pathogenesis including macrophages, monocytes, neutrophils, dendritic cells, and lymphocytes, but also in non-immune cells, including endothelial cells, cardio-myocytes, fibroblasts, osteoblasts, and epithelial cells [106, 128, 131, 132, 136, 138, 139].
Even though NLRP3 inflammasome is the best-characterized canonical inflammasome, the intracellular upstream mechanisms and stimuli activating this inflammasome are not well-defined [140]. Nevertheless, many studies proposed several structurally and chemically different stimuli involved in the upstream activation steps of NLRP3 inflammasome. These stimuli include PAMPs, DAMPs, ionic (potassium [K+], chloride [Cl–], and calcium [Ca2+]) flux, reactive oxygen species (ROS) produced after mitochondrial dysfunction (mtROS), lysosomal damages, metabolic changes, and trans-Golgi disassembly-associated particles [7, 128, 131]. Moreover, there are no concluding studies clearly demonstrating a direct interaction between at least one of the aforementioned stimuli and a component of the NLRP3 inflammasome. Saying that, the clear mechanism initiating NLRP3 inflammasome activation still remains hypothetical, thus to be deeply investigated and confirmed. However, it is thought that NLRP3 senses common cascade cellular events induced by these proposed stimuli.
Early studies have proposed a two-signal model for NLRP3 inflammasome activation. Indeed, the first priming signal of NLRP3 inflammasome response is to induce the expression of NLRP3 inflammasome protein components because of low expression levels in a variety of cell types [141,142,143]. The interaction between extracellular PRRs and PAMPs/DAMPs induces the transcriptional activity of intracellular inflammatory signaling molecules, such as nuclear factor (NF)-kB and activator protein-1 (AP-1) to upregulate the production level of NLRP3 inflammasome molecules [144]. The next triggering signal is initiated to assemble NLRP3 inflammasome complexes and result in the signal transduction cascades to ultimately active caspase-1. Enzymatically active caspase-1 cleaves the pro-inflammatory cytokine (IL-1β and IL-18) to yield bioactive cytokine and naturally, autoinhibited gasdermin-D (GSDMD) to release the cellular content into the extracellular space by membrane pores formation [Regulation by post-transcriptional modifications A plethora of studies report that the NLRP3 inflammasome can be activated independently of NLRP3 transcription, suggesting that the priming process has other essential regulatory mechanisms. Deubiquitination and phosphorylation of NLRP3 are the two PTMs that are involved in the regulation of NLRP3 inflammasome activation and inhibition. Upon priming or in presence of NLRP3 inflammasome stimuli such as LPSs, ubiquitination of the macrophage NLRP3 proteins is inhibited to promote the NLRP3 oligomerization and inflammasome complex formation and NLRP3 proteins remain deubiquitinated during the inflammatory process, until the pathogen is cleared. Inhibition of NLRP3 ubiquitination is mediated by SCFFBXL2 E3 ligase (FBXO3), which targets and senses FBXL2, an endogenous mediator of NALP3 degradation NLRP3 via Trp-73 interaction. In fact, the knockdown of FBXO3, known to interact with ubiquitin, yields in low release of IL-18 and IL-1β [201], involved in inflammatory response. Once the inflammatory process has cleared the infection, to maintain immune homeostasis and avoid detrimental effects, E3 ubiquitin ligase TRIM31 and dopamine directly bind to NLRP3 protein and attenuate NLRP3 inflammasome activation by promoting Lys-48-linked polyubiquitination and proteasomal degradation of oligomerized NLRP3 proteins [202, 203]. Similarly, it has been demonstrated that stimulation through TLR4 by ligand LPSs activates MAPK8 (JUN N-terminal kinase-1 [JNK1]), which directly interacts with human NLRP3 Ser-198 (Ser-194 residue in mice) and induces phosphorylation of NLRP3 protein [204]. Another study [205] demonstrated that, at the Golgi apparatus, protein kinase D (PKD) interacts with human NLRP3 Ser-295 (Ser291 in mouse NLRP3) and phosphorylates NLRP3, promoting NLRP3 oligomerization. Consequently, JNK1- and PKD-mediated NLRP3 phosphorylation induces NLRP3 deubiquitination, facilitates its oligomerization and self-association, and the subsequent inflammasome assembly. However, it has been demonstrated that NLRP3 phosphorylation can also suppress or repress activation of NLRP3 inflammasome. Indeed, PYD has been found to interact directly with human NLRP3 Ser-5 (Ser-3 in mouse NLRP3), which in consequence prevents NLRP3 inflammasome activation [206, 207]. We are tempted to believe that the phosphorylation of NLRP3 protein selectively depends on whether inflammatory needs to be induced or repressed, in presence of absence of NLRP3 stimuli. Therefore, both in resting cells and after inflammatory-associated infection clearance, PYD might selectively interact directly with human NLRP3 Ser-5 (Ser-3 in mouse NLRP3) and repress NLRP3 inflammasome activation. In the same manner, bile acids and prostaglandin E2 induce protein kinase A (PKA)-mediated phosphorylation and thus repress activation of NLRP3 inflammasome [128, 131]. Besides deubiquitination and phosphorylation of NLRP3 protein, another PTMs, including sumoylations have been identified to be involved in regulation of the NLRP3 inflammasome activity [208]. Indeed, upon priming by NLRP3 inflammasome stimuli, the sumoylation of macrophage NLRP3 is repressed by sentrin-specific protease 6 (SENP6) and SENP7, which induces NLRP3 oligomerization and promotes NLRP3 inflammasome complex formation. However, prior to and after NLRP3 activation, NLRP3 is sumoylated by the E3 SUMO protein ligase MUL1 (also known as MAPL), restraining or negatively regulating NLRP3 inflammasome activation. As described above, the main proteins that are involved in forming an activated NLRP3 inflammasome complex to induce inflammatory responses include NLRP3, ASC, and the enzyme pro-caspase-1 (Fig. 5). However, it has been found that other intracellular proteins might interact with NLRP3 protein and modulate activity of NLRP3 inflammasome. Specifically, Pyrin-only proteins (POPs, aka PYDC proteins), CARD-only proteins (COPs), chaperone heat shock protein 90 (Hsp90) and its co-chaperone SGT1, guanylate-binding protein 5 (GBP5), RNA-dependent protein kinase (PKR), migration inhibitory factor (MIF), thioredoxin-interacting protein (TXNIP), microtubule-affinity regulating kinase 4 (MARK4), and NEK7, have been reported to activate and/or inhibit activation of NLRP3 inflammasome, based on the need cell [128, 131, 209]. In resting macrophages, Hsp90 with its cofactor SGT1 are found to interact with NLRP3 forming a complex that protects NLRP3 from degradation and keeps it in an inactive form but ready to be sensed by NLRP3 stimuli. In presence of intracellular ROS induced by NLRP3 stimuli, TXNIP and especially NEK7 interact with NLRP3 and induce NLRP3 inflammasome activation. Similarly, in response to ATP, nigericin, and bacteria-associated stimuli, GBP5 and PKR have been reported to prime NLRP inflammasome activation, even though their roles in the activation of NLRP inflammasomes are controversial [195, 196, 210,211,212,213,214,215]. Reversely, to down-regulate or to negatively regulate NLRP3 inflammatory response after pathogen clearance, it was demonstrated that POP1 and POP2 (among the 4 POPs (POP 1–4)) bind to ASC and inhibit NLRP3-ASC interaction [128]. In this context, it is the last product of the activated NLRP3 inflammasome complex pathway (IL-1β) which has a feedback effect (similar to an allosteric effect) on POP1 and POP2 to downregulate NLRP3 inflammasome activation or prevent its over activation (reviewed in [128, 209]). While activation of NLRP3 inflammasome should be associated with host defense against infections and infection relief, its aberrant or improper activation, inactivation and dysfunction during infection, or lack of shutdown after infection release (hyper-activation) are detrimental to health and associated with several health disorders (Fig. 2 and Table 1). Mutations within NRLP3 inflammasome compounds have been the main causes of dysregulation of NLRP3 inflammasome activation and responsible for inflammatory response-associated diseases. The gain-of-function mutation(s) within Nlrp3 gene have been the first cause of NLRP3 inflammasome dysregulation and associated with inflammatory disorders, one of which being CAPS, a rare condition covering familial cold autoinflammatory syndrome (FCAS, MIM 120100), also known as familial cold urticaria (FCU), Muckle–Wells syndrome (MWS), and neonatal onset multi-systemic inflammatory disease (NOMID) [216]. CAPS is thought to be symptomatically characterized by chronic fever, rashes, inflamed eyes, arthritis, swelling, headaches, deafness and amyloidosis [217]. Indeed, mutations identified within Nlrp3 genes (including CIAS1 that encodes NACHT and LRR [216] and NLRP3-encoding residues adjacent to Ser295 [218]), were associated with aberrant activation of NLRP3 inflammasome, which is the main cause of CAPS. In Alzheimer’s disease, accumulation of fibrillar peptide amyloid-β after phagocytosis releases cathepsin B that is sensed by NLRP3 (and at a lesser extent NLRP1) and induces activation of NLRP1 and 3 inflammasomes [219], which in turn have been found to worsen Alzheimer’s disease patient conditions [220,221,222,223]. To prevent such activation in patients suffering from Alzheimer’s disease, inhibition of NLRP3 and NLRP1 has been found to be promising as it promotes non-phlogistic clearance of amyloid-β and improves cognitive functions [224, 225]. Similarly, NLRP3 inflammasome-associated inflammatory response is not always benefic such as in patients suffering from Parkinson’s disease where NLRP3 inflammasome activation has been associated with neurodegeneration, and that knocking down NLRP3 improve health conditions [105]. A similar detrimental effect of NLRP3 inflammasome activation has been demonstrated in traumatic brain injury that causes neuroinflammation. Indeed, a few hours after traumatic brain injury, NRLP inflammatory mediators, including NLRP3 are upregulated, increasing the activation of inflammation and release of pro-inflammatory cytokines [226]. Studies have demonstrated that patients who have suffered from traumatic brain injury have an increased risk for chronic inflammatory activation in neurons and consequently neurodegenerative diseases [227,228,229]. These chronic neuro-inflammatory responses are thought to increase hyperphosphorylation of tau protein and amyloid-β, two precursors that worsen Alzheimer’s disease through NLRP3 inflammasome activation. NLRP3 inflammasome activation and of IL-1β, IL-1 and IL-18 are thought to play a detrimental role in multiple sclerosis (an autoimmune neurodegenerative disorder caused by infiltration of autoreactive T-cells inside the central nervous system through a weakened blood–brain barrier) by facilitating immune cell infiltration and promoting excessive inflammatory response, which in turn aggravates conditions in multiple sclerosis patients [20, 105]. Dysregulation of NLRP3 inflammasome activation, specifically chronic activation of NLRP3 inflammasome, has been involved in pathogenesis of rheumatoid arthritis, gouty arthritis, diabetes and worsening disease conditions. In fact, release of particles in rheumatoid arthritis (pentaxin 3 and its ligand C1q), gout (uric acid crystals), and diabetes significantly over-activates NLRP3 inflammasome [230, 231], which contributes in development of these peripheral inflammatory diseases [232]. Finally, the activation of NLRP inflammasomes has a protective role in different types of cancer; however, its over-activation has been associated with a destructive and promoting role for cancer development [105].Regulation by NLRP3-interacting proteins
NLRP3 inflammasome dysfunction and associated diseases
NLRP6 inflammasomes
NLRP6 inflammasome and its role in health and innate immunity
The role of NLRP6 to form a cytosolic inflammasome complex and to be involved in innate immunity has long been a debate and non-conclusive. Recent reports have concluded that NLRP6 is a standalone protein forming inflammasome, as they have shown that NLRP6 is able to form an inflammasome complex and cleaves precursor of and release IL-1β and IL-18 during microbial infection [109, 233, 234]. Specifically, NLRP6 was initially shown to activate both caspase-1 and NF-κB. This double activity of NLRP6 inflammasome, unique in NLRP inflammasomes, has been associated with a broad range of physiological functions, including modulation of the host-microbial interface [110, 235, 236], host defense against pathogens [237, 238], and inhibition of carcinogenesis [109] and neuro-inflammation [239]. NLRP6 is one of the major NLRP inflammasomes found in the intestine and liver and was discovered to protect from colitis and ensure homeostasis of intestinal and gut microbiota, and regulate intestinal antiviral innate immunity [235, 236]. Moreover, NLRP6 inflammasome is also found in the kidney tissues and neuronal, lymphocyte, and bone marrow-derives cells [240, 241]. Studies on NLRP6 inflammasome have gained interest just recently because of the significant role NLRP6 inflammasome plays in regulating inflammation and host defenses in specific tissues and organs. In bone marrow-derived macrophages, NLRP6 inflammasome suppresses inflammatory signaling [238]. In goblet cells, the intestinal mucosal epithelial cells that mainly synthesize mucus, NLRP6 inflammasome mediates mucus secretion upon stimulation by TLR ligands or microbiota-associated metabolites, thereby regulating the intestine bacterial population diversity and preventing their abnormal increase or intestine invasiveness by new bacteria [237, 242, 243]. In response to infection by viruses, such as RNA viruses, NLRP6 inflammasome aims to regulate the expression of numerous IFN-stimulated genes through the mitochondrial adaptor protein MAVS [235] (Fig. 2). Relatively newly discovered amongst the NLRP inflammasomes, NLRP6 inflammasome activation mechanism is not fully understood yet; researches are still on going.
Structural and functional organization of NLRP6 inflammasome
NLRP6 protein, initially known as PYPAF5 protein, has been found to recruit and assemble with ACS protein, caspase-1, or caspase-11 to form an inflammasome complex, mediating proteolysis-based maturation and secretion of IL-8 and IL-1β [109, 234, 244]. NLRP6 inflammasome consists of consists of the NLRP6 protein (the sensor), ASC (the adaptor) and, pro-caspase enzyme (the effector) (Fig. 1). NLRP6 protein is highly expressed in intestinal epithelial goblet cells, where the activated NLRP6 inflammasome complex is responsible for regulating the gut microbiome composition and involved in gastrointestinal inflammatory [235, 236]. NLRP6 protein is also expressed in lungs, liver, and tubular epithelium of kidneys [109, 233, 245, 246]. In buccal cavity cells, especially in gingiva and periodontium cells, NLRP6 inflammasome plays a central role in homeostasis regulation [247, 248]. This distribution of NLRP6 through tissues and cells lies on the multiplicity of NLRP6 transcriptional promotors, which are tissue- or cells-selectively up-regulated. The Human Nlrp6 gene carries three alternative promotors. The first promotor is located in exon 1 (in the 5’ UTR) and modulates expression of NLRP6 in intestine. The second promotor is located in exon 2 (within the PYD domain) and modulates expression of NLRP6 in kidney, liver, lung, neurons, and spleen. The third promotor is located in exon 3 (in the region between PYD and NBD or NACHT) [249]. However, in mice, the Nlrp6 gene carries two tissue-specific promotors; one is located in the exon 1 (in the 185 base-pair of 5’UTR) and promotes expression of NLRP6 in intestinal tissues, and the other is a result of the alternative splicing of exon 1, but located in 1749 base-pair 5’UTR, and promotes expression of NLRP6 in kidney and liver [249, 250].
Like other NLRPs, NLRP6 consists of three domains (Fig. 1): i) the PYD, which interacts with ACS, is essential to initiate NLRP6 complex formation [251], ii) the NBD (or the NACHT) in the center of the complex followed by iii) the LRR domain that is involved in sensing microbial PAMPs and/or DAMPs (review in [245]). Unlike NLRP3 PYD which cannot promote NLRP3 ACSPYD polymerization alone but only complexed with NBD (NACHT), NLRP6 PYD is a stronger nucleator and, in somewhat high concentration, is able to promote NLRP6 ACSPYD polymerization. Specifically, the NLRP6 PYD alone is capable of auto-assembling into filamentous structures followed by large conformational changes and uses PYD-PYD interactions to recruit the ASC adaptor [251]. However, it was shown that the complex formed by PYD-NBD of NLRP6 is stronger in NLRP6 polymerization than the PYD domain alone, which suggests that this assembling process is strengthened by the PYD fused to its NBD (NACHT) domain of NLRP6.
Molecular mechanism of NLRP6 inflammasome activation
Despite the crucial role played by NLRP6 inflammasome in host defense regulation especially in intestinal microbiota and innate immune signaling in myeloid cells as detailed hereinbefore, its activation molecular mechanism is yet to be fully decrypted, as it has only been recently discovered. Nevertheless, currently available studies have made a non-negligible contribution to exploring and detailing the molecular mechanisms of NLRP6 inflammasome assembly and activation.
From current reports, the activation mechanism of NLRP6 inflammasome is similar to the canonical pathway of NLRP3 activation, described as a two-step model, including a priming step and an activation step. Like with NLRP3 inflammasome activation mechanism, the NLRP6 inflammasome-priming step is triggered by NLRP6 stimuli and lies on induction and transcriptional regulation of the Nlrp6 gene and the expression and oligomerization of NLRP6 protein. The activation step is required to achieve NLRP6 inflammasome assembly, which is followed by inflammatory response characterized by pyroptosis (Fig. 6).
The mechanisms of human NLRP6 inflammasome activation. The activation of NLRP6 inflammasome obey a two-steps mechanism: a priming and an activation. In the priming step, induction of the Nlrp6 gene transcription and other NLRP6 inflammasome components is triggered by TNF‐α, viral and bacterial PAMPs/DAMPs, and/or the peroxisome proliferator‐activated receptor-γ (PPAR‐γ). Once translated, NLRP6 inflammasome is activated by dsARN from RNA virus and LPS, and occurs through NLRP6 recruitment of ASC and Casp1. The activated NLRP6 inflammasome activates IL-1β, and IL-18 from their respective precursors (pro-IL-1β, and pro-IL-18, respectively) by catalytic cleavage. NLRP6 inflammasome also catalyses digestion of GSDMD into GSDMD-C and GSDMD-N that forms gasdermin membrane pore, which yields to pyroptosis. IL-1β and IL-18 cytokines are release out of the cells to promote pro-inflammatory responses. Besides, the NLRP6 protein is also found in the cytosol in low level and is autoinhibited in quiescent cell condition. In this condition, LTA from Gram + bacteria induce an indirect non-canonical activation of the NLRP6 inflammasome. LTA activates caspase-11 (involved in the non-canonical inflammasome activation pathway) which trigger activation of NLRP3/6 inflammasomes, through production of ions flux (specifically K+ efflux via GSDMD pores), which in turn activate caspase-1 and release IL-1β and IL-18
Signal 1: priming the NLRP6 inflammasome
The upstream NLRP6 inflammasome stimuli that mainly trigger the up-regulation of Nlrp6 expression include pro-inflammatory signals and metabolites such as TNF‐α or viral and bacterial PAMPs/DAMPs [235, 245, 245, 250] (Fig. 6). Few molecular regulators have been found to prime and induce up-regulation of NLRP6. Upon immune cell exposition to microbial and metabolic NLRP6 stimuli, the peroxisome proliferator‐activated receptor-γ (PPAR‐γ), a transcription factor involved in metabolic regulation [252], and its agonist rosiglitazone [253], induce transcription of Nlrp6 and high expression of NLRP6 protein. More precisely, PPARγ directly binds to the PPAR‐γ-retinoid X receptor-α (PPAR‐γ-RXR-α) at the promoter region of Nlrp6 and induces transcription and expression of NLRP6 [253]. Besides, Roux-en-Y gastric bypass (RYGB) has also been shown to prime NLRP6, through up-regulation of NLRP6 expression upon exposition of intestine cells to microbiota-related metabolites, taurine, and histamine, resulting from intestinal permeability due to obesity [254]. Moreover, as the first priming signal, in interesting study by Hara et al. [240] reported that Gram-positive bacteria, such as Listeria monocytogenes, induce up-regulation transcription of Nlrp6, expression of both NLRP6 and caspase-11, and then activation of NLRP6 inflammasome. Specifically, the lipoteichoic acid from L. monocytogenes induces activation of PPARγ but more specifically up-regulates type 1 interferon (IFN-1) signaling in macrophages, which in turn induces the expression of NLRP6 and caspase-11 [237, 240] (Fig. 6).
Signal 2: activating the NLRP6 inflammasome
Once translated, NLRP6 activation requires specific triggers to induce assembly into a typical inflammasome complex, which will activate pro-IL-1β and pro-IL-18 from their respective precursors. These triggers are known as a second signal or signal 2. Amongst the second signals activating NLRP6, lipoteichoic acid (LTA) has been found to serve as a ligand to interact with NLRP6 and activate inflammasome via a signaling cascade of protein recruitment. In fact, LTA binds to the expressed NLRP6 through its LRR domain, promoting ASC poly-oligomerization. Particularly, LTA-based activation of NLRP6 induces the non-canonical NLRP6 inflammasome activation pathway. Indeed, functional NLRP6 activation by LTA activates does not activate caspase-1 directly, but rather does activate caspase-11 [240], which is involved in the non-canonical inflammasome activation pathway. The caspase-11-associated activation of non-canonical pathway triggers activation of NLRP3/6 inflammasomes, through production of ions flux (specifically K+ efflux via GSDMD pores), which in turn activate caspase-1 and release IL-1β and IL-18 [250].
Recent reports have highlighted a novel NLRP6 inflammasome activation model, where LTA but specifically viral dsRNA can promote NLRP6 to form a liquid–liquid phase separation (LLPS) which is associated with NLRP6 activation [250]. NLRP6 would form LLPS thanks to its polybasic domain located within the NACHT domain (350–354) and after phase separation, ASC will solidify NLRP6 aggregates and will yield NLRP6 inflammasome activation [250, 255].
NLRP6 inflammasome can also be auto-activated in presence of inflammasome stimuli. As described in a review by Ghimire et al. [256] it has been demonstrated that PYD and CARD filaments display prion-like properties that facilitate polymerizations of ASC into filamentous structures leading to activation of ASC-dependent NLRP6 inflammasome [56, 129]. In more detail, ASC-dependent NLRP6 inflammasome activation involves a two nucleation-induced polymerization steps, including i) a first ASCPYD filament nucleation by NLRPYD through a PYD-PYD interaction leading to polymerization of ASC, and ii) a second nucleation of caspase-1 CARD filaments by the polymerized ASCCARD through a CARD-CARD interaction that directly activates caspase-1, which induces release of IL-1β and IL-18 [256]. Besides, LPSs of Gram-negative bacteria also activate translated NLRP6. In-vitro studies reported that LPSs can interact directly with NLRP6, promoting conformational change and subsequently inducing NLRP6 homo-dimerization [257] (Fig. 6).
NLRP6 inflammasome regulation and dysfunction
Regulation of NLRP6 inflammasome activation
Like for NLRP1 and NLRP3, NLRP6 inflammasome activation needs to be highly and timely regulated, as its chronic imbalanced activation, including inactivation when needed or hyper-activation (excessive inflammation), yields several inflammatory and metabolic diseases [107] (Table 1). As previously described, expression of NLRP6 and activation of NLRP6 inflammasome is up-regulated at the transcriptional and post-transcriptional levels. PPAR‐γ is so far though to be one of the first up regulators of Nlrp6 transcription. Besides, in quiescent cells or in absence of ligand activators, NLRP6 inflammasome activation is prevented by the closed conformation of the LRR and NACHT domains of NLR; this inhibition is known as auto-inhibition [256, 258].
Furthermore, recent reports demonstrated that deubiquitinase Cyld plays an important role in preventing uncontrolled and excessive activation of NLRP6 inflammasome. Interestingly, Cyld deubiquinates NLRP6 by destroying the bound to K63 that maintains NLRP6 ubiquitinated, thereby preventing NLRP6 from recruiting ASC [259]. Specific LPSs, including γ-D-glutamyl-meso-diaminopimelic acid (iE‐DAP, an agonist of NOD1), Pam3CysSerLys4 (Pam3CSK4, a synthetic triacylated LPS agonist of TLR1/TLR2), and muramyl dipeptide (MDP) were demonstrated to up-regulate NLPR6 inflammasome activation [243]. Beyond bacterial product, which additionally includes LTA as a strong up-regulator of NLRP6 inflammasome activation together with taurine, histamine, and spermine, ATP-dependent RNA helicase DEAH (Asp‐Glu‐Ala‐His) box helicase 15 (Dhx15) has been thought to be sensed by NLRP6 and enhances activation of NLRP6 inflammasome [235].
After infection clearance, the NLRP6 inflammasome needs to be down-regulated. Interestingly, it has been demonstrated that Nlrp6 transcription and NLRP6 expression are down-regulated by miRNA‐331‐3p after cerebral hemorrhage [260], which stops the inflammatory response and restores cell homeostasis. Likewise, while miR-650 is thought to promote NLRP6 inflammasome-related apoptosis, NLRP6 itself acts as an auto-inhibitor by decreasing the apoptosis increased by the effect of miR-650 through a direct binding of the Nlrp6 3’UTR and miR-650, to restore cell homeostasis after infection clearance [261]. Besides the described regulation mechanism of NLRP6 activation, further studies would provide more details about, and with NLRP6 inflammasome implications in health and disease.
NLRP6 inflammasome dysfunction and associated diseases
When not properly regulated, dysregulation of NLRP6 inflammasome activation manifests several diseases, which include familial Mediterranean fever caused by mutations in the pyrin‐coding gene MEFV, or cryopyrin‐associated periodic syndrome, caused by point mutations in Nlrp6 gene [107] (Table 1). Moreover, because NLRP6 has mainly been associated with gut microbiota protection, dysregulation of NLRP activation has been associated with colitis and persistent gut infection [109, 110]. Besides, NLRP6 inflammasome dysfunctions have been associated with adrenomedullin (ADM) loci and male essential hypertension, which suggests that NLRP6 inflammasome and potentially other NLRP inflammasome dysregulation promote pathogenesis of essential hypertension [262].
NLRP7 inflammasome
NLRP7 inflammasomes and its role in health and innate immunity
So far, NLRP7 protein has only been described in various human cells and tissues, including lymphocytes, monocytic cells, lung, spleen, thymus, ovaries, and oocytes, but not in rodents’ [263], where it has been found to assemble into an inflammasome complex and induce pyroptosis [264, 265]. However, the exact role of NLRP7 inflammasome in health is currently controversial. On the first hand, NLRP7 is associated with a pro-inflammatory protective function. NLRP7 has been found to sense bacterial lipopeptides (PAMPs) and form an activated inflammasome complex, promoting activation of caspase-1, maturation and release of IL-1β and IL-18, which induce a protective inflammatory response against intracellular bacterial replication [265]. Moreover, the protective role of NLRP7 inflammasome has also been described in ovaries and oocytes, where it promotes embryonic development, though the mechanism modulating this protective role is unknown [263, 266]. On another hand, activation of NLRP7 inflammasome has been associated with an anti-inflammatory role [264, 265]. Specifically, NLRP7 can prevent secretion of IL-1β and inhibit the effector function mediated by the NLRP3 inflammasome [234, 267]. Note that a mutation in Nlrp7 gene found in peripheral blood mononuclear cells from hydatidiform mole patients has been strongly associated with a reduced secretion of IL-1β upon LPS treatment, which was not the case in healthy individual cells. Taken together, it is fair to assume that mutation(s) in NLRP7 might induce NLRP7 inflammasome anti-inflammatory activation response and might be specific to tissues or cells. Complementarily, because the anti-inflammatory response associated with NLRP7 inflammasome might be caused regardless of Nlrp7 mutation, it would be coherent to assume, as suggested by Zeng et al. [268], that NLRP7 would negatively regulate inflammation in quiescent cells, while upon stimulation by PAMPs and DAMPs, NLRP7 would promote inflammasome assembly, activation of caspase-1, and release of pro-inflammatory cytokines. Whatever the case is, in-depth studies are required to elucidate the role of NLRP7 inflammasome in inflammatory response.
Structural and functional organization of NLRP7 inflammasome
NLRP7 inflammasome has been found to form bona-fide inflammasome complex triggered by acylated bacterial lipopeptides [264, 265]. It consists of the sensor NLRP7, the adaptor ASC, the effector adaptor, and the effector pro-caspase-1 (Fig. 1). NLRP7 senses stimuli (such as ATP) through its NACHT domain to form inflammasome and remain ubiquitinated to regulate its functions [269]. However, its ability to form inflammasome complex remain to be elusive, as several studies report contradictory functions of NLRP7 inflammasome in health and diseases. Overall, the mechanism leading to NLRP7 inflammasome activation remains to be elucidated.
NLRP7 inflammasome-associated diseases
Like NLRP3 and NLRP6 inflammasomes, NLRP7 inflammasome activation needs to be highly regulated regardless of its functions (pro- or ant-inflammatory), to insure and maintain the associated protective functions. It has been reported that mutations occurring in Nlrp7 gene are associated with hydatidiform mole, a gestational trophoblastic disease that develops during the early stage of pregnancy and is responsible for a nonviable fetus [111, 266, 270]. Interestingly, these loss-of-function mutations that lead to hydatidiform mole phenotypes are mainly located within the LRR domain of Nlrp7 gene [266], which suggests that LRR play a central role in NLRP7 inflammasome activation. Moreover, it has recently been suggested that a decreased level of NLPR7 protein in trophoblasts disrupts the methylation of DNA and CpG and promotes differentiation of trophoblast lineages, which in turn causes typical trophoblast hypertrophy. This function has only been described for NLRP7 inflammasome and indicates that Nlrp7 is involved in chromatin programming [112].
Other NLRP inflammasomes
Besides NLRP1, the newly identified CARD8, NLRP3, NLRP6, and NLRP7, known to form bona-fide inflammasomes, several other NLRP family proteins have been reported as sensors in inflammasome complex formation. For instance, NLRP2, NLRP9, NLRP10, and NLRP12 have been found to sense inflammasome stimuli, use ACS as adaptor molecules, and induce release of pro-inflammatory cytokines. However, for some of them, their roles in health are still unclear and their activation mechanisms are to be investigated.
NLRP2 inflammasome
NLRP2 (aka NALP2, PYPAF2, NBS1, PAN1, and CLR19.9) is expressed in many human cells, including astrocytes [271] and proximal tubular epithelial cells specifically in people suffering from inflammatory diseases [272]. The ability of NLRP2 to form an inflammasome has recently been suggested, though little is known about how NLRP2 specifically mediates to assemble into and form an activated inflammasome. As with all NLRP inflammasome structural organization, the NLRP2 inflammasome is a multiprotein complex composed of the sensor NLRP2, the adaptor ASC, and the caspase-1. It is thought that, like NLRP1, NLRP2 inflammasome would assemble through a direct interaction of NLRP2 with ASC and subsequently with CARD8 domain, which interacts with and regulates caspase-1 activation [273,274,275]. In human astrocytes, the DAMP such as ATP mediates NLRP2 inflammasome activation, leading to the processing of inflammatory caspase-1 and interleukin-1β (IL-1β). In addition, it was found that NLRP2 could interact with the P2X7 receptor and the pannexin-1 channel, leading to NLRP2 inflammasome activation [271]. Moreover, NLRP2 positively up-regulates pro-fibrotic mediator expression and NF-κB activation, by modulating the p65 NF-κB phosphorylation, but down-regulates that expression of several interferon-inducible genes. However, overexpression of NLRP2 in proximal tubular epithelial cells hampers the apoptotic reaction [272]. Besides, kynurenine, a tryptophan metabolite produced as a biomarker in the immune dysfunction of depression, was recently shown to activate NLRP2 inflammasome in astrocytes. An increased level of kynurenine in the mouse hippocampus together with the presence of ATP is associated with NLRP2 inflammasome activation, which is characterized by expression of caspase-1 and release of IL-1β. More interestingly, a treatment with kynurenine promotes the translocation of NF-κB to the nucleus and its binding to NLRP2 promotor, which subsequently induces an increased NLRP2 transcription, modulating inflammasome activation [276]. This recent study by Zhang et al. [276] evidenced that NLRP2 inflammasome plays an important role in depressive behavioral, as it participates in inflammatory immune response. Knocking-out kynurenine and/or NLRP2 hampers inflammation and restores homeostasis, which suggests that drugs targeting kynurenine or NLRP2 in depression-like behaviors would relieve from these states. NLRP2 is also expressed in human brain vascular pericytes, together with NLRP1, NLRP3, NLRP5, NLRP9, and NLRP10. In these brain cells, NLRP2 inflammasome activation occurs through the non-canonical activation pathway triggered by intracellular LPS or E. coli. Bacteria [277].
NLRP9 inflammasome
Similar to NLRP1, which is represented by only one gene in humans (hNLRP1), but four paralogues in rodents (mNLRP1a-d), NLRP9 is represented by only one gene in humans (hNLRP9) but three isoforms in rodents (mNLRP1a-c) [25] and are mainly expressed in reproductive organs [278]. Specifically expressed in human, murine, and bovine oocysts, ovaries, and testes, NLRP9 expression has been associated with preimplantation and development of embryos, and lack of all mNLRP9 isoforms [279, 280] (not a single one [281, 282]) in mice hampers embryonic preimplantation and development.
While its role in reproductive cells is somewhat elaborated, NLRP9 is one of the less- and the last characterized NLRP family proteins in terms of protein-forming inflammasome. Thus, the role of NLRP9 in infectious and inflammatory diseases is still elusive. Recent studies have reported that NLRP9 could initiate assembly into a protective activated inflammasome involved in host immune defense against proliferation of infectious diseases, especially in the intestine. Specifically, except in reproductive cells, mNLRP9b (not mNLRP9a or mNLRPb) was also found to be highly expressed in the ileum, where it was associated with caspase-1 cleavage and IL-18 release upon rotavirus infection, as mNLRP9b-depleted mice showed elevated viral load and severe pathogenesis compared to wild-type mice harboring mNLRP9b [283]. This suggests that NLRP9 can potentially form a bona-fide inflammasome that triggers a protective inflammatory response against pathogens.
In regards to what precedes, conclusions on the molecular and structural mechanisms of NLRP9 activation would be based on mNLRP9b investigations, as there are few or no studies on NLRP9 inflammasome mechanisms of assembly. Yet, how NLRP9 inflammasome assembles remains largely unknown. In their study, Zhu et al. [283] demonstrated that mNLRP9b serves as a sensor for rotavirus infection to trigger NLRP9 inflammasome formation. Specifically, mNLRPb indirectly senses rotavirus RNA through an intermediate binding with DExH-box helicase (DHX)9, an RNA helicase that has a high binding affinity with dsRNA, here rotavirus, and forms a complex. In absence of DHX9 in experimental challenge cells with rotavirus, low production of IL-18 together with resistance to pyroptosis were observed. It is necessary to note that DHX9 is unable to bind host RNA, which could trigger NLRP9 inflammasome-associated auto-inflammatory response but specifically recognizes viral dsRNA, via a mechanism that is still unknown [284]. Moreover, which domain of NLRP9 that binds to DHX9 is also unrevealed. Moreover, how the NLRP9-DXH9-RNA complex recruits ASC and other monomeric subunits necessary for assembly or how the formed complex induces activation of NLRP9 inflammasome, which protects against rotavirus needs to be elucidated. Furthermore, as it is shown for rotavirus in intestine, more studies are needed to explore the NLRP9 effect in other cells and organs.
Conversely, while NLRP9 inflammasome could protect against rotavirus in intestine, another study has demonstrated that mNLRP9b inflammasome is involved in enhancement of acute lung injury [285]. Indeed, wild-type mice carrying mNLRPb gene showed a higher neutrophilic inflammatory response and a decreased survival rate compared with mNLRP9b-depleted mice, which suggests that NLRP9 inflammasome activation, like other NLRP inflammasomes, can be detrimental to health. More interestingly, as recently reviewed [286], besides lung injury [285], NLRP9 inflammasome is involved in the occurrence or the enhancement of many other diseases, including chronic childhood arthritis (systemic-onset juvenile idiopathic arthritis [287]), multiple sclerosis [288], Alzheimer’s disease [289], urothelial carcinoma [290], and Helicobacter pylori-associated infection [291]. Note that these last cited NLRP9 inflammasome-associated diseases are related to a mutation in NLPR9 gene that may cause a dysfunction of NLRP inflammasome and its aberrant activation.
NLRP10 inflammasome
Also known as NOD8, PAN5, or PYNOD, NLRP10 protein was described for the first time by Wang et al. [292] and has been found in rodent immune and human cells, including epithelial cells, keratinocytes, macrophages, dendritic, and T cells [246]. However, the expression of NLRP10 in each of these cells and environment seems different and associated with different variable, but contradictory functions. In fact, NLRP10 is the only protein from NLRP protein family that does not have the same structural features as other NLRP proteins known to form inflammasome complex, as it lacks the LRR domain involved in homotypic CARD-CARD domain interactions required to recruit the enzyme pro-caspase-1 for a typical inflammasome complex assembly. Thus, NLRP10 protein only consists of PYD and NBD (NACHT) domains. This characteristic suggests that NLRP10 might have an NLRP10 inflammasome-independent function, as NLRP10 protein may not act like a signal sensor of PAMPs and DAMPs and be involved in forming inflammasome, but rather like a probable regulator or an adaptor [293]. In other words, NLRP10 looks more like a regulator rather than an inflammasome-associated sensor, and may not form a bona-fide inflammasome, unless another domain is involved in recruiting pro-caspase-1 to allow assembly into an active inflammasome with effect on caspase maturation and interleukin release. Notably, Lech et al. [246] and Imamura et al. [294] demonstrated the anti-inflammatory and inflammasome-independent function of NLRP10 in innate immunity. They showed that NLRP10 protein negatively regulates other inflammasome activation and inflammasome-associated cell death. Specifically, they described that NLRP10 inhibits ASC-mediated NF-κB activation and prevents the release of IL-1β by hampering the caspase-1-mediated maturation of IL-1β. These activities have been also shown in other reports and are attributed to the NACHT domain (and to a lesser extent PYD) of NLRP10, which interacts with caspase-1 through ASC of other inflammasomes [292, 293].
However, these findings may not result in a general conclusion because, it has also been surprisingly and contrarily shown that in other cells different from the human epithelial cells used in the above description, NLRP10 may have an inflammasome-dependent activity, meaning NLRP10 is able to form an inflammasome. Notably, while the negative regulation of NLRP10 was shown by others, reporting inhibition of NLRP3-associated normal canonical activation and IL-1β production in mouse DCs carrying NLRP10 [294], the presence of NLRP10 in transgenic mouse macrophages has no negative effects in ASC aggregate formation, nor does it have negative regulation in caspase-1-mediated maturation of IL-1β. Also, recent studies by Prochnicki et al. [295] and Zheng et al. [296]have shown that NLRP10 can assemble into a bona-fide inflammasome in differentiated human keratinocytes and is involved in monitoring mitochondrial integrity in an mtDNA-independent manner, as 3M3-FBS triggers NLRP10 inflammasome activation via mitochondrial disruption. This suggests that NLRP10 expression seems to depend on cell-type and its function is cellular environment- and signaling pathways-dependent [296,297,298]. Therefore, more investigations are required for a better understanding of the NLRP3 inflammasome-dependent and independent functions in innate immunity and whether, or not, NLRP10 forms a functional inflammasome. Such studies are relevant to facilitating new innate immune anti-inflammatory interventional strategies [296].
NLRP12 inflammasome
NLRP12 protein (aka Monarch-1 or Pypaf7) is mainly expressed in immune cells, including bone marrow-derived dendritic cells, granulocytes, macrophages, and neutrophils [299, 300]. Like NLRP10, NLRP12 was described as a negative regulator for normal canonical activation and IL-1β secretion in activated B-cell signaling through interaction with IRAK1 that prevents its accumulation. Similarly, NLRP12 down-regulates non-canonical activation through interaction with TRAF3 that promotes the degradation of NF-κB-inducing kinase [301,302,303]. In hematopoietic and non-hematopoietic stem cell subsets, NLRP12 has been found to act like an inflammatory response modulator [301]. In addition, it has been found that NLRP12, together with NLRP10, can negatively regulate adaptive immunity [304].
Besides being an immune response regulator and like other NLRP family proteins (except NLRP10), NLRP12 has been found to inflammasome-forming component. This role of NLRP12 in forming inflammasome and the inflammasome-associated function was evidenced in part from infection with Yersinia pestis, in which NLRP12 inflammasome induced activation of caspase-1 and release of pro-inflammatory cytokines, including IL-1β and IL-18, and was associated with protection against Yersinia pestis infection [305]. However, in the in-vivo infection model, NLRP12 and NLRP3 inflammasomes are concomitantly required to provide an anti-infectious resistance against Yersinia pestis, which suggests that in some infection setups, activation of divers NLRP family proteins or NLRP inflammasomes might provide an optimal protection again microbial antigens [306].
NLRP12 was amongst the first NLRP family proteins to be described together with NLRP1 and NLRP3, and displays structural similarities with NLRP3 [299, 300]. However, the structural and functional organization of NLRP12 inflammasome needs to be studied. Even though NLRP12 inflammasome activation mechanism is still unknown, its activation process should be highly regulated to ensure health and homeostasis. Indeed, dysregulation of NLRP12 inflammasome activation, in part caused by numerous identified mutations (above 20 mutation types [307, 308]), has been associated with health disorders and systemic inflammatory diseases [307,308,309]. Patients displaying CAPS-associated symptoms have been shown to carry a set of NLRP12 but not always NLRP3 mutations, considered to dysregulate NLRP12 inflammasome activation and trigger the NLRP12-associated auto-inflammatory diseases (NLRP12-AID) [307,308,309]. However, it is important to note that certain of these NLRP12 mutations do not always compromise the protective pro-inflammatory effect of NLRP12 inflammasome but rather promote a gain-of-function, as they are associated with increased caspase activation and enhanced IL-1β secretion. Therefore, these conflicting roles of NLRP12 [306], including i) the NLRP12-assocciated protective pro-inflammatory function, ii) the NLRP12 mutation-associated diseases triggering function, and iii) the NLRP12 negative regulation role described above might have rendered initial attempts for anti-IL-1 therapy difficult and unsuccessful, and may contribute to explain mechanisms underlying resistance to anti-IL-1 therapy observed in patients with CAPS [310]. Thus, to provide a successful therapy against such a scenario, further in-depth studies are highly needed.
Therapeutic strategies against pathogenic NLRP inflammasomes
As previously described, aberrant NLRP inflammasome activation and gain-of-function mutations have been associated with the development and enhancement of numerous metabolic, auto-inflammatory, autoimmune, and neurodegenerative diseases. The currently used immunosuppressive and anti-inflammatory treatment, which include cyclosporine, steroids, methotrexate, and general anti-TNF-a therapy allows to treat severe cases of inflammatory diseases [304, 311]. However, a challenge rises in such a way that the immunosuppressive and anti-inflammatory treatment may hamper activation of a normal protective immune response that is not associated with disease-specific pathological mechanisms. Thus, to overcome this challenge, it is suggested to thoroughly understand the difference between activation mechanisms of the proper induction of protective immune response and that is specifically leading to diseases, as this would allow development of aberrant inflammasome-specific drugs that could not hamper immune response induced to clear infection [304].
Fortunately, to pave this way, promising therapeutics that specifically and selectively inhibit aberrant NLRP inflammasome activation in inflammatory diseases have been developed and proven effective. As summarized in Table 2, these therapeutics mainly target and inhibit NLRP inflammasome products, including IL-1β and IL-18, and hamper the NLRP inflammasome activation ability of sensor stimuli [312]. For example, the inhibitors in CAPS that inhibit downstream pro-inflammatory cytokine also contribute to reducing the pathogenic effect of CAPS by blocking inflammasome-independent but CAPS-dependent pyroptosis released DAMPs that are produced to induce more CAPS-related pathological inflammation [313]. The best example of inhibitors that play such roles includes canakinumab and anakinra, a monoclonal anti-IL-1β antibody and a recombinant IL-1 receptor antagonist (IL-1RA), respectively. Canakinumab and IL-1RA anakinra are currently approved for treating certain forms of arthritis, CAPS, and Mediterranean fever, and they are effective in reducing cardiovascular events in atherosclerosis patients [314]. As highlighted by Bulte et al. [304], these inhibitors may still hamper induced protective immunity upon infection.
Besides, recent research discovered and characterized many inflammasome inhibitors that directly target NLPR genes or NLRP inflammasome complex-associated components, blocking their oligomerization, the further inflammasome activation pathway, and the subsequent release of pro-inflammatory cytokines. For instance, the 3,4-methylenedioxy-β-nitrostyrene (MNS) that has been demonstrated to treat inflammasome activation-induced inflammatory bowel disease (IBD), prevents NLRP3-mediated ASC speck formation and oligomerization and inhibits NLRP3 ATPase activity by binding to NLRP3 LRR and NLRP3 NACTH [324, 325]. Tranilast, another direct NLRP3 inhibitor, inhibits NLRP3 oligomerization by directly binding to NLRP3 NACHT domain and prevents NLRP3 inflammasome assembly [317, 335, 336]. Moreover, VI-16 blocks oligomerization of NLRP3 and activation of TXNIP-dependent NLRP3 inflammasome. Table 2 shows other example inhibitors that directly target NLRPs or NLRPs-associated complexes.. These NLRP inflammasome inhibitors tend to be the best against pathogenic inflammatory responses because, unlike the previous, they inhibit the formation of inflammasome complex, so blocking any downstream activity. Drugs that inhibit NLRP inflammasome products would not prevent inflammasome complex formation. Therefore, treatment with such inhibitors would be a long-term process and difficult to stop, as stop** it would still lead to production of downstream pro-inflammatory cytokines by activated inflammasomes, thus compromising the health state. This later might, however not be observed when treating with inhibitors that directly target NLPRs or NLRP components. In fact, we hypothesized that, besides mutations-based aberrant inflammation, blocking NLRP oligomerization for further aberrant inflammasome complex activation would help the inflammasome regulation system to restore proper regulation of NLRP inflammasome activation. It would be therefore suitable to study whether short-term treatment with direct NLRP inflammasome inhibitors permanently restores cellular homeostasis and ameliorates health conditions in aberrant inflammasome activation-associated diseases.
The way to deal with improper NLRP inflammasome activation and the associated pathological conditions is still long. Note that almost, if not all current NLRP inflammasome inhibitors considered under clinical trials target NLRP3 inflammasome (Table 2 and review in [304]), unless the intravenous immunoglobulin (IVIg) which is an FDA-approved drug against NLRP1 and NLRP3 inflammasome activation [348]. Moreover, as presented in Table 2, most of the discovered or developed inhibitors of NLRP inflammasome activation are only towards NLRP3 inflammasome, but not other described NLRP inflammasomes. This suggests that besides the promising potential to inhibit aberrant activation of NLRP3 inflammasome, considerable progress is required, especially in develo** other NLRP inflammasome-specific drugs than those targeting NLRP3 inflammasome [351].
Concluding remarks and future perspectives
Among the NLRP inflammasomes, NLRP3 inflammasome is the most highly described, with its molecular mechanism of assembly and activation well enough elaborated so far. Its role in health preservation and occurrence of auto-inflammatory, autoimmune, and neurological diseases and aggravation of these conditions is also well described. It is noteworthy that most studies in develo** pharmacological inhibitors to treat pathological inflammasome activation are mainly directed against NLRP3 inflammasome, for which promising drugs against NLRP3 inflammasome-associated diseases are in advanced stages of clinical trial. Although encouraging, as these studies/results allow management of NLRP3 inflammasome activation and the associated diseases, huge effort is still required for both understanding of NLRP3 inflammasome mechanisms of modulation and other NLRP inflammasomes mechanisms of activation and modulation, and for the development of specific inflammasome therapeutic inhibitors.
Many parameters have hampered the studies on the mechanism of assembly of certain inflammasomes, such as NLRP1 and NLRP9 inflammasomes. The molecular mechanisms of NLRP1 assembly and activation were poorly characterized because of the lack of common activators in human and mouse models, and because of the inherent structural differences in hNLRP1 and mNLRP1s [25, 31,32,33,34]. Current reports have revealed numerous perturbations and molecular entities related to NLRP1 biology (e.g., dsRNA, viral 3C proteases and reductive stress). Notably, NLRP1 stimuli can be divided into two distinct groups based on pathogens signals and danger signals. One group is pathogen signals which contain viral 3C proteases, viral dsRNA, KSHV-encoded ORF45, lethal anthrax toxin, IpaH7.8 of Shigella flexneri and T. gondii infections. UVB, O3, ATP, peptide accumulation, metabolic inhibitors, and reductive and ribotoxic stress belong to the other group of danger signals. Interestingly, the wide variety of agonists has unraveled the pivotal role of NLRP1 inflammasome in cellular homeostasis and host defense responses.
Furthermore, although there are some unresolved mysteries regarding NLRP inflammasome activation, recent studies have provided much about NLRP1, NLRP6, and NLRP7 receptors that can detect diverse pathogens or dangerous activities. Yet, several questions arise to understand the molecular mechanisms of NLRP inflammasome activation. Indeed, regarding NLRP1, one interesting question raised by these observations includes how dsRNA triggers a conformational shift of the N-terminal region of NLRP1 to destabilize the ternary complex remains elusive. Another outstanding question consists of how DPP8/9 inhibitors precisely affect the CARD8-DPP9 ternary complex to activate the NLRP1 inflammasome in cells. Although keratinocytes and AML (Acute Myeloid Leukemia) cells express NLRP1 and CARD8, the molecular mechanism of how VbP activates NLRP1 inflammasome in keratinocytes, whereas CARD8 sense VbP in AML cells, remains unknown. Moreover, understanding how some NLRP inflammasomes are activated and modulated, post-activation mechanisms that trigger pyroptosis might serve as therapeutic targets to modulate or inhibit pathologic NLRP inflammasome activation. Also, it has been thought that expression of NLRPs is associated with either pathologic or benefic effect/response in health or disease based on the tissue; thus more studies are needed to understand what drives these NLRP cell-specific differences of function.
As for NLRP3 activation pathways, studies characterizing the upstream and downstream pathways of NLRP1, NLRP6, NLRP6, and NLRP7 inflammasome activation have suggested important roles in human diseases (Fig. 2 and Table 1). Because the aberrant activation of NLRP inflammasomes is associated with several auto-inflammatory diseases, and their inhibition could be a useful pharmacological approach for managing chronic inflammatory disorders. Many studies have demonstrated that potential NLRP1 inhibitors significantly prevent NLRP1 inflammasome complex formation [351]. In addition, as presented in Table 2, the blockade of NLRP inflammasome downstream signaling, such as the IL-1β receptor, could be a suitable pharmacological approach for treating immune-mediated inflammatory diseases [352].
In the effort to develop therapeutics against aberrant activation of NLRP inflammasomes, it is important to note that most of the current therapeutic inhibitors (Table 2) do not directly target NLRP inflammasome components, but are specific to the final products of inflammasomes such as IL-1β, IL-18, NF-kB signaling, and so on. Thus, we are tempted to suggest that more effort should be put into develo** therapeutics that target NLRP inflammasome components. Targeting NLPR inflammasome components, if possible, would be more beneficial because it would reduce the random blockade of normal production of IL-1β and IL-18 for example, which are not always harmful or induced by NLRP inflammasome activation. In addition, such targets could or may be directed to mutant NLRPs that lead to aberrant inflammasome activation, and therefore would solve the question of the limit between the harmful and the beneficial inflammasome activation and how to react accordingly.
Overall, these recent advances in our understanding of the mechanisms of NLRP inflammasome activation offer insight into inflammasome assembly and signaling. Further exploration is needed to gain insight into the complex activation mechanism of other NLRP inflammasomes and how their dysfunction is associated with human diseases.
Availability of data and materials
Not applicable.
References
Janeway, C.A., Jr. & Medzhitov, R. Innate immune recognition. Annu Rev Immunol. 2002;20197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359.
Leal, V.N.C. & Pontillo, A. Canonical Inflammasomes. Methods Mol Biol. 2023;26961–27. https://doi.org/10.1007/978-1-0716-3350-2_1.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32. https://doi.org/10.1016/j.cell.2010.01.040.
Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–26. https://doi.org/10.1038/nature06246.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. https://doi.org/10.1016/s1097-2765(02)00599-3.
Rathinam VA, Fitzgerald KA. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165(4):792–800. https://doi.org/10.1016/j.cell.2016.03.046.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20. https://doi.org/10.1038/nri.2016.58.
Asare Y, Shnipova M, Zivkovic L, Schlegl C, Tosato F, Aronova A, et al. IKKbeta binds NLRP3 providing a shortcut to inflammasome activation for rapid immune responses. Signal Transduct Target Ther. 2022;7(1):355. https://doi.org/10.1038/s41392-022-01189-3.
Ghait, M., Duduskar, S.N., Rooney, M., Hafner, N., Reng, L., Gohrig, B. et al. The non-canonical inflammasome activators Caspase-4 and Caspase-5 are differentially regulated during immunosuppression-associated organ damage. Front Immunol. 2023;141239474. https://doi.org/10.3389/fimmu.2023.1239474.
Sharma, A.K. & Ismail, N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells. 2023;12(22). https://doi.org/10.3390/cells12222597.
Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci. 2017;130(23):3955–63. https://doi.org/10.1242/jcs.207365.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. https://doi.org/10.1038/nm.3893.
Ding, J. & Shao, F. SnapShot: The Noncanonical Inflammasome. Cell. 2017;168(3):544–544 e1. https://doi.org/10.1016/j.cell.2017.01.008.
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–92. https://doi.org/10.1038/nature13683.
Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, et al. Interleukin-1beta Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep. 2018;24(6):1425–33. https://doi.org/10.1016/j.celrep.2018.07.027.
Carty, M., Kearney, J., Shanahan, K.A., Hams, E., Sugisawa, R., Connolly, D. et al. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity. 2019;50(6):1412–1424 e6. https://doi.org/10.1016/j.immuni.2019.04.005.
**a, S., Hollingsworth, L.R.t. & Wu, H. Mechanism and Regulation of Gasdermin-Mediated Cell Death. Cold Spring Harb Perspect Biol. 2020;12(3). https://doi.org/10.1101/cshperspect.a036400.
Zhang Y, Dong Z, Song W. NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct Target Ther. 2020;5(1):37. https://doi.org/10.1038/s41392-020-0145-7.
Moloudizargari, M., Moradkhani, F., Asghari, N., Fallah, M., Asghari, M.H., Moghadamnia, A.A. et al. NLRP inflammasome as a key role player in the pathogenesis of environmental toxicants. Life Sci. 2019;231116585. https://doi.org/10.1016/j.lfs.2019.116585.
Chen L, Cao SQ, Lin ZM, He SJ, Zuo JP. NOD-like receptors in autoimmune diseases. Acta Pharmacol Sin. 2021;42(11):1742–56. https://doi.org/10.1038/s41401-020-00603-2.
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27519–50. https://doi.org/10.1146/annurev.immunol.021908.132612.
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. https://doi.org/10.1038/nri2691.
Shen, Y., Qian, L., Luo, H., Li, X., Ruan, Y., Fan, R. et al. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022;12(8). https://doi.org/10.3390/brainsci12081057.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–8. https://doi.org/10.1038/nature18629.
Tian, X., Pascal, G. & Monget, P. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol Biol. 2009;9202. https://doi.org/10.1186/1471-2148-9-202.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. https://doi.org/10.1038/s41591-019-0675-0.
Ma Q. Pharmacological Inhibition of the NLRP3 Inflammasome: Structure, Molecular Activation, and Inhibitor-NLRP3 Interaction. Pharmacol Rev. 2023;75(3):487–520. https://doi.org/10.1124/pharmrev.122.000629.
van den Berg, D.F. & Te Velde, A.A. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front Immunol. 2020;111580. https://doi.org/10.3389/fimmu.2020.01580.
Raman KS, Matsubara JA. Dysregulation of the NLRP3 Inflammasome in Diabetic Retinopathy and Potential Therapeutic Targets. Ocul Immunol Inflamm. 2022;30(2):470–8. https://doi.org/10.1080/09273948.2020.1811350.
Sun Y, Reddy P. CH 18 - Intracellular sensors of immunity and allogeneic hematopoietic stem cell transplantation. In: Socié G, Blazar BR, editors. Immune Biology of Allogeneic Hematopoietic Stem Cell Transplantation. San Diego: Academic Press; 2013. p. 425–47.
Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38(2):240–4. https://doi.org/10.1038/ng1724.
Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28(3):285–7. https://doi.org/10.1016/j.immuni.2008.02.005.
Sastalla, I., Crown, D., Masters, S.L., McKenzie, A., Leppla, S.H. & Moayeri, M. Transcriptional analysis of the three Nlrp1 paralogs in mice. BMC Genomics. 2013;14188. https://doi.org/10.1186/1471-2164-14-188.
Lilue J, Doran AG, Fiddes IT, Abrudan M, Armstrong J, Bennett R, et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat Genet. 2018;50(11):1574–83. https://doi.org/10.1038/s41588-018-0223-8.
Dawson, H.D., Smith, A.D., Chen, C. & Urban, J.F., Jr. An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Vet Microbiol. 2017;2022–15. https://doi.org/10.1016/j.vetmic.2016.05.013.
Jha S, Ting JP. Chapter 12 - Inflammasome effector functions: a Tale of Fire and Ice. In: Pablo Pelegrin, editor. Inflammasome Biology. Academic Press. 2023. p. 179–204. https://doi.org/10.1016/B978-0-323-91802-2.00031-1. https://www.sciencedirect.com/science/article/pii/B9780323918022000311.
He H, Wang W, Li L, Zhang X, Shi H, Chen J, et al. Activation of the NLRP1 Inflammasome and Its Role in Transmissible Gastroenteritis Coronavirus Infection. J Virol. 2023;97(6): e0058923. https://doi.org/10.1128/jvi.00589-23.
Tsu, B.V., Beierschmitt, C., Ryan, A.P., Agarwal, R., Mitchell, P.S. & Daugherty, M.D. Diverse viral proteases activate the NLRP1 inflammasome. Elife. 2021;10. https://doi.org/10.7554/eLife.60609.
Bauernfried, S., Scherr, M.J., Pichlmair, A., Duderstadt, K.E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021;371(6528). https://doi.org/10.1126/science.abd0811.
Li, J., Yan, M., Zhang, Y., Feng, C., Wang, H., Wang, C. et al. Meta-analysis of the association between NLRP1 polymorphisms and the susceptibility to vitiligo and associated autoimmune diseases. Oncotarget. 2017;8(50):88179–88188. https://doi.org/10.18632/oncotarget.21165.
** T, Curry J, Smith P, Jiang J, **ao TS. Structure of the NLRP1 caspase recruitment domain suggests potential mechanisms for its association with procaspase-1. Proteins. 2013;81(7):1266–70. https://doi.org/10.1002/prot.24287.
Maharana, J. Elucidating the interfaces involved in CARD-CARD interactions mediated by NLRP1 and Caspase-1 using molecular dynamics simulation. J Mol Graph Model. 2018;807–14. https://doi.org/10.1016/j.jmgm.2017.12.016.
Zhong, F.L., Mamai, O., Sborgi, L., Boussofara, L., Hopkins, R., Robinson, K. et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167(1):187–202 e17. https://doi.org/10.1016/j.cell.2016.09.001.
Xu Z, Zhou Y, Liu M, Ma H, Sun L, Zahid A, et al. Homotypic CARD-CARD interaction is critical for the activation of NLRP1 inflammasome. Cell Death Dis. 2021;12(1):57. https://doi.org/10.1038/s41419-020-03342-8.
Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287(30):25030–7. https://doi.org/10.1074/jbc.M112.378323.
D’Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS ONE. 2011;6(11): e27396. https://doi.org/10.1371/journal.pone.0027396.
Janssens S, Tinel A. The PIDDosome, DNA-damage-induced apoptosis and beyond. Cell Death Differ. 2012;19(1):13–20. https://doi.org/10.1038/cdd.2011.162.
Chavarria-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev. 2015;265(1):22–34. https://doi.org/10.1111/imr.12283.
Frew BC, Joag VR, Mogridge J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8(4): e1002659. https://doi.org/10.1371/journal.ppat.1002659.
Chui AJ, Okondo MC, Rao SD, Gai K, Griswold AR, Johnson DC, et al. N-terminal degradation activates the NLRP1B inflammasome. Science. 2019;364(6435):82–5. https://doi.org/10.1126/science.aau1208.
Sandstrom, A., Mitchell, P.S., Goers, L., Mu, E.W., Lesser, C.F. & Vance, R.E. Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes SM. Science. 2019;364(6435). https://doi.org/10.1126/science.aau1330.
Robinson KS, Toh GA, Rozario P, Chua R, Bauernfried S, Sun Z, et al. ZAKalpha-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science. 2022;377(6603):328–35. https://doi.org/10.1126/science.abl6324.
Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8(6):471–83. https://doi.org/10.1016/j.chom.2010.11.007.
Bauernfried, S. & Hornung, V. Human NLRP1: From the shadows to center stage. J Exp Med. 2022;219(1). https://doi.org/10.1084/jem.20211405.
** Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–25. https://doi.org/10.1056/NEJMoa061592.
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–206. https://doi.org/10.1016/j.cell.2014.02.008.
Lu A, Li Y, Schmidt FI, Yin Q, Chen S, Fu TM, et al. Molecular basis of caspase-1 polymerization and its inhibition by a new cap** mechanism. Nat Struct Mol Biol. 2016;23(5):416–25. https://doi.org/10.1038/nsmb.3199.
Li Y, Fu TM, Lu A, Witt K, Ruan J, Shen C, et al. Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc Natl Acad Sci U S A. 2018;115(43):10845–52. https://doi.org/10.1073/pnas.1810524115.
Sborgi L, Ravotti F, Dandey VP, Dick MS, Mazur A, Reckel S, et al. Structure and assembly of the mouse ASC inflammasome by combined NMR spectroscopy and cryo-electron microscopy. Proc Natl Acad Sci U S A. 2015;112(43):13237–42. https://doi.org/10.1073/pnas.1507579112.
Gong Q, Robinson K, Xu C, Huynh PT, Chong KHC, Tan EYJ, et al. Structural basis for distinct inflammasome complex assembly by human NLRP1 and CARD8. Nat Commun. 2021;12(1):188. https://doi.org/10.1038/s41467-020-20319-5.
Robert Hollingsworth L, David L, Li Y, Griswold AR, Ruan J, Sharif H, et al. Mechanism of filament formation in UPA-promoted CARD8 and NLRP1 inflammasomes. Nat Commun. 2021;12(1):189. https://doi.org/10.1038/s41467-020-20320-y.
Friedlander, A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261(16):7123–6. https://www.ncbi.nlm.nih.gov/pubmed/3711080
Moayeri M, Sastalla I, Leppla SH. Anthrax and the inflammasome. Microbes Infect. 2012;14(5):392–400. https://doi.org/10.1016/j.micinf.2011.12.005.
Varshavsky A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 2011;20(8):1298–345. https://doi.org/10.1002/pro.666.
Sriram SM, Kim BY, Kwon YT. The N-end rule pathway: emerging functions and molecular principles of substrate recognition. Nat Rev Mol Cell Biol. 2011;12(11):735–47. https://doi.org/10.1038/nrm3217.
Xu, H., Shi, J., Gao, H., Liu, Y., Yang, Z., Shao, F. et al. The N-end rule ubiquitin ligase UBR2 mediates NLRP1B inflammasome activation by anthrax lethal toxin. EMBO J. 2019;38(13):e101996. https://doi.org/10.15252/embj.2019101996.
Sandstrom, A., Mitchell, P.S., Goers, L., Mu, E.W., Lesser, C.F. & Vance, R.E. Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science. 2019;364(6435). https://doi.org/10.1126/science.aau1330.
Robinson, K.S., Teo, D.E.T., Tan, K.S., Toh, G.A., Ong, H.H., Lim, C.K. et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science. 2020;370(6521). https://doi.org/10.1126/science.aay2002.
Planes, R., Pinilla, M., Santoni, K., Hessel, A., Passemar, C., Lay, K. et al. Human NLRP1 is a sensor of pathogenic coronavirus 3CL proteases in lung epithelial cells. Mol Cell. 2022;82(13):2385–2400 e9. https://doi.org/10.1016/j.molcel.2022.04.033.
Yang X, Zhou J, Liu C, Qu Y, Wang W, **ao MZX, et al. KSHV-encoded ORF45 activates human NLRP1 inflammasome. Nat Immunol. 2022;23(6):916–26. https://doi.org/10.1038/s41590-022-01199-x.
Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, et al. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog. 2014;10(3): e1003927. https://doi.org/10.1371/journal.ppat.1003927.
Ewald SE, Chavarria-Smith J, Boothroyd JC. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun. 2014;82(1):460–8. https://doi.org/10.1128/IAI.01170-13.
Gorfu, G., Cirelli, K.M., Melo, M.B., Mayer-Barber, K., Crown, D., Koller, B.H. et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio. 2014;5(1). https://doi.org/10.1128/mBio.01117-13.
Liao KC, Mogridge J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect Immun. 2013;81(2):570–9. https://doi.org/10.1128/IAI.01003-12.
Orth-He EL, Huang HC, Rao SD, Wang Q, Chen Q, O’Mara CM, et al. Protein folding stress potentiates NLRP1 and CARD8 inflammasome activation. Cell Rep. 2023;42(1): 111965. https://doi.org/10.1016/j.celrep.2022.111965.
Ball, D.P., Tsamouri, L.P., Wang, A.E., Huang, H.C., Warren, C.D., Wang, Q. et al. Oxidized thioredoxin-1 restrains the NLRP1 inflammasome. Sci Immunol. 2022;7(77):eabm7200. https://doi.org/10.1126/sciimmunol.abm7200.
Wang Q, Hsiao JC, Yardeny N, Huang HC, O’Mara CM, Orth-He EL, et al. The NLRP1 and CARD8 inflammasomes detect reductive stress. Cell Rep. 2023;42(1): 111966. https://doi.org/10.1016/j.celrep.2022.111966.
Meihe, L., Shan, G., Minchao, K., **aoling, W., Peng, A., **li, W. et al. The Ferroptosis-NLRP1 Inflammasome: The Vicious Cycle of an Adverse Pregnancy. Front Cell Dev Biol. 2021;9707959. https://doi.org/10.3389/fcell.2021.707959.
Ferrara, F., Cordone, V., Pecorelli, A., Benedusi, M., Pambianchi, E., Guiotto, A. et al. Ubiquitination as a key regulatory mechanism for O3-induced cutaneous redox inflammasome activation. Redox Biol. 2022;56102440. https://doi.org/10.1016/j.redox.2022.102440.
Burian M, Yazdi AS. NLRP1 Is the Key Inflammasome in Primary Human Keratinocytes. J Invest Dermatol. 2018;138(12):2507–10. https://doi.org/10.1016/j.jid.2018.08.004.
Lee, T.A., Huang, Y.T., Hsiao, P.F., Chiu, L.Y., Chern, S.R. & Wu, N.L. Critical roles of irradiance in the regulation of UVB-induced inflammasome activation and skin inflammation in human skin keratinocytes. J Photochem Photobiol B. 2022;226112373. https://doi.org/10.1016/j.jphotobiol.2021.112373.
Robinson KS, et al. ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science. 2022;377:328–35. https://doi.org/10.1126/science.abl6324.
Jenster, L.M., Lange, K.E., Normann, S., vom Hemdt, A., Wuerth, J.D., Schiffelers, L.D.J. et al. P38 kinases mediate NLRP1 inflammasome activation after ribotoxic stress response and virus infection. J Exp Med. 2023;220(1). https://doi.org/10.1084/jem.20220837.
Adams S, Miller GT, Jesson MI, Watanabe T, Jones B, Wallner BP. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 2004;64(15):5471–80. https://doi.org/10.1158/0008-5472.CAN-04-0447.
Walsh MP, Duncan B, Larabee S, Krauss A, Davis JP, Cui Y, et al. Val-boroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of established murine tumors. PLoS ONE. 2013;8(3): e58860. https://doi.org/10.1371/journal.pone.0058860.
Okondo, M.C., Rao, S.D., Taabazuing, C.Y., Chui, A.J., Poplawski, S.E., Johnson, D.C. et al. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol. 2018;25(3):262–267 e5. https://doi.org/10.1016/j.chembiol.2017.12.013.
Zhong FL, Robinson K, Teo DET, Tan KY, Lim C, Harapas CR, et al. Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. J Biol Chem. 2018;293(49):18864–78. https://doi.org/10.1074/jbc.RA118.004350.
Huang M, Zhang X, Toh GA, Gong Q, Wang J, Han Z, et al. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. 2021;592(7856):773–7. https://doi.org/10.1038/s41586-021-03320-w.
Hollingsworth LR, Sharif H, Griswold AR, Fontana P, Mintseris J, Dagbay KB, et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 2021;592(7856):778–83. https://doi.org/10.1038/s41586-021-03350-4.
Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8(3): e1002638. https://doi.org/10.1371/journal.ppat.1002638.
Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129(1):45–56. https://doi.org/10.1016/j.cell.2007.01.045.
Gerlic M, Faustin B, Postigo A, Yu EC, Proell M, Gombosuren N, et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc Natl Acad Sci U S A. 2013;110(19):7808–13. https://doi.org/10.1073/pnas.1215995110.
Ozretic, P., da Silva Filho, M.I., Catalano, C., Sokolovic, I., Vukic-Dugac, A., Sutic, M. et al. Association of NLRP1 Coding Polymorphism with Lung Function and Serum IL-1beta Concentration in Patients Diagnosed with Chronic Obstructive Pulmonary Disease (COPD). Genes (Basel). 2019;10(10). https://doi.org/10.3390/genes10100783.
Pontillo A, Girardelli M, Kamada AJ, Pancotto JA, Donadi EA, Crovella S, et al. Polimorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity. 2012;45(4):271–8. https://doi.org/10.3109/08916934.2011.637532.
Magitta NF, Boe Wolff AS, Johansson S, Skinningsrud B, Lie BA, Myhr KM, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 2009;10(2):120–4. https://doi.org/10.1038/gene.2008.85.
** Y, Birlea SA, Fain PR, Spritz RA. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol. 2007;127(11):2558–62. https://doi.org/10.1038/sj.jid.5700953.
Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, ** Y, et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci U S A. 2013;110(8):2952–6. https://doi.org/10.1073/pnas.1222808110.
De Iudicibus S, Stocco G, Martelossi S, Londero M, Ebner E, Pontillo A, et al. Genetic predictors of glucocorticoid response in pediatric patients with inflammatory bowel diseases. J Clin Gastroenterol. 2011;45(1):e1-7. https://doi.org/10.1097/MCG.0b013e3181e8ae93.
Fenini, G., Karakaya, T., Hennig, P., Di Filippo, M. & Beer, H.D. The NLRP1 Inflammasome in Human Skin and Beyond. Int J Mol Sci. 2020;21(13). https://doi.org/10.3390/ijms21134788.
Scheufele C, Ezaldein HH, Rothbaum R, Delost GR. Multiple self-healing palmoplantar carcinoma: An aberrance of the inflammasome. JAAD Case Rep. 2019;5(3):261–3. https://doi.org/10.1016/j.jdcr.2019.01.001.
Yang Q, Yu C, Yang Z, Wei Q, Mu K, Zhang Y, et al. Deregulated NLRP3 and NLRP1 Inflammasomes and Their Correlations with Disease Activity in Systemic Lupus Erythematosus. J Rheumatol. 2014;41(3):444–52. https://doi.org/10.3899/jrheum.130310.
Costa, F.R.C., Leite, J.A., Rassi, D.M., da Silva, J.F., Elias-Oliveira, J., Guimarães, J.B. et al. NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes. Cell Reports. 2021;35(8). https://doi.org/10.1016/j.celrep.2021.109176.
Sun, X., **a, Y., Liu, Y., Wang, Y., Luo, S., Lin, J. et al. Polymorphisms in NLRP1 Gene Are Associated with Type 1 Diabetes. Journal of Diabetes Research. 2019;20191–9. https://doi.org/10.1155/2019/7405120.
Drutman SB, Haerynck F, Zhong FL, Hum D, Hernandez NJ, Belkaya S, et al. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc Natl Acad Sci U S A. 2019;116(38):19055–63. https://doi.org/10.1073/pnas.1906184116.
Blevins, H.M., Xu, Y., Biby, S. & Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front Aging Neurosci. 2022;14879021. https://doi.org/10.3389/fnagi.2022.879021.
Moltrasio, C., Romagnuolo, M. & Marzano, A.V. NLRP3 inflammasome and NLRP3-related autoinflammatory diseases: From cryopyrin function to targeted therapies. Front Immunol. 2022;131007705. https://doi.org/10.3389/fimmu.2022.1007705.
Broderick, L., De Nardo, D., Franklin, B.S., Hoffman, H.M. & Latz, E. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10395–424. https://doi.org/10.1146/annurev-pathol-012414-040431.
Toubai T, Fujiwara H, Rossi C, Riwes M, Tamaki H, Zajac C, et al. Host NLRP6 exacerbates graft-versus-host disease independent of gut microbial composition. Nat Microbiol. 2019;4(5):800–12. https://doi.org/10.1038/s41564-019-0373-1.
Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186(12):7187–94. https://doi.org/10.4049/jimmunol.1100412.
Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–59. https://doi.org/10.1016/j.cell.2014.01.026.
Neidhart M. Chapter 14 - DNA Methylation in Growth Retardation. In: Neidhart M, editor. DNA Methylation and Complex Human Disease. Oxford: Academic Press; 2016. p. 241–59.
Mahadevan S, Wen S, Wan YW, Peng HH, Otta S, Liu Z, et al. NLRP7 affects trophoblast lineage differentiation, binds to overexpressed YY1 and alters CpG methylation. Hum Mol Genet. 2014;23(3):706–16. https://doi.org/10.1093/hmg/ddt457.
Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis. 2017;76(7):1191–8. https://doi.org/10.1136/annrheumdis-2016-210021.
Okondo MC, Johnson DC, Sridharan R, Go EB, Chui AJ, Wang MS, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13(1):46–53. https://doi.org/10.1038/nchembio.2229.
Taabazuing, C.Y., Okondo, M.C. & Bachovchin, D.A. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol. 2017;24(4):507–514 e4. https://doi.org/10.1016/j.chembiol.2017.03.009.
Linder, A., Bauernfried, S., Cheng, Y., Albanese, M., Jung, C., Keppler, O.T. et al. CARD8 inflammasome activation triggers pyroptosis in human T cells. EMBO J. 2020;39(19):e105071. https://doi.org/10.15252/embj.2020105071.
Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med. 2018;24(8):1151–6. https://doi.org/10.1038/s41591-018-0082-y.
Johnson DC, Okondo MC, Orth EL, Rao SD, Huang HC, Ball DP, et al. DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes. Cell Death Dis. 2020;11(8):628. https://doi.org/10.1038/s41419-020-02865-4.
Wang, Q., Gao, H., Clark, K.M., Mugisha, C.S., Davis, K., Tang, J.P. et al. CARD8 is an inflammasome sensor for HIV-1 protease activity. Science. 2021;371(6535). https://doi.org/10.1126/science.abe1707.
Sharif, H., Hollingsworth, L.R., Griswold, A.R., Hsiao, J.C., Wang, Q., Bachovchin, D.A. et al. Dipeptidyl peptidase 9 sets a threshold for CARD8 inflammasome formation by sequestering its active C-terminal fragment. Immunity. 2021;54(7):1392–1404 e10. https://doi.org/10.1016/j.immuni.2021.04.024.
Xu, Z., Deng, S., Huang, Y., Yang, Y., Sun, L., Liu, H. et al. The CARD8 T60 variant associates with NLRP1 and negatively regulates its activation. Front Immunol. 2022;131047922. https://doi.org/10.3389/fimmu.2022.1047922.
Figueiredo A, Moore KL, Mak J, Sluis-Cremer N, de Bethune MP, Tachedjian G. Potent nonnucleoside reverse transcriptase inhibitors target HIV-1 Gag-Pol. PLoS Pathog. 2006;2(11): e119. https://doi.org/10.1371/journal.ppat.0020119.
Clark KM, Kim JG, Wang Q, Gao H, Presti RM, Shan L. Chemical inhibition of DPP9 sensitizes the CARD8 inflammasome in HIV-1-infected cells. Nat Chem Biol. 2022. https://doi.org/10.1038/s41589-022-01182-5.
Tsu, B.V., Agarwal, R., Gokhale, N.S., Kulsuptrakul, J., Ryan, A.P., Castro, L.K. et al. Host specific sensing of coronaviruses and picornaviruses by the CARD8 inflammasome. bioRxiv. 2022. https://doi.org/10.1101/2022.09.21.508960.
Griswold AR, Ball DP, Bhattacharjee A, Chui AJ, Rao SD, Taabazuing CY, et al. DPP9’s Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation. ACS Chem Biol. 2019;14(11):2424–9. https://doi.org/10.1021/acschembio.9b00462.
Rao SD, Chen Q, Wang Q, Orth-He EL, Saoi M, Griswold AR, et al. M24B aminopeptidase inhibitors selectively activate the CARD8 inflammasome. Nat Chem Biol. 2022;18(5):565–74. https://doi.org/10.1038/s41589-021-00964-7.
Yaron JR, Gangaraju S, Rao MY, Kong X, Zhang L, Su F, et al. K(+) regulates Ca(2+) to drive inflammasome signaling: dynamic visualization of ion flux in live cells. Cell Death Dis. 2015;6(10): e1954. https://doi.org/10.1038/cddis.2015.277.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. https://doi.org/10.1038/s41577-019-0165-0.
Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156(6):1207–22. https://doi.org/10.1016/j.cell.2014.01.063.
Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19(10):610–21. https://doi.org/10.1038/s41583-018-0055-7.
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20(13). https://doi.org/10.3390/ijms20133328.
Takahashi M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res. 2022;118(2):372–85. https://doi.org/10.1093/cvr/cvab010.
Schmid-Burgk JL, Chauhan D, Schmidt T, Ebert TS, Reinhardt J, Endl E, et al. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J Biol Chem. 2016;291(1):103–9. https://doi.org/10.1074/jbc.C115.700492.
He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–7. https://doi.org/10.1038/nature16959.
Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17(3):250–8. https://doi.org/10.1038/ni.3333.
Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215(3):827–40. https://doi.org/10.1084/jem.20172222.
Schmidt FI, Lu A, Chen JW, Ruan J, Tang C, Wu H, et al. A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J Exp Med. 2016;213(5):771–90. https://doi.org/10.1084/jem.20151790.
Zhong, Y., Kinio, A. & Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013;4333. https://doi.org/10.3389/fimmu.2013.00333.
** Y, Fu J. Novel Insights Into the NLRP 3 Inflammasome in Atherosclerosis. J Am Heart Assoc. 2019;8(12): e012219. https://doi.org/10.1161/JAHA.119.012219.
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. https://doi.org/10.1038/s41419-019-1413-8.
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–91. https://doi.org/10.4049/jimmunol.0901363.
Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183(2):792–6. https://doi.org/10.4049/jimmunol.0900173.
Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186(4):2529–34. https://doi.org/10.4049/jimmunol.1002720.
Yi, Y.S., Son, Y.J., Ryou, C., Sung, G.H., Kim, J.H. & Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014270302. https://doi.org/10.1155/2014/270302.
Evavold, C.L., Ruan, J., Tan, Y., **a, S., Wu, H. & Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity. 2018;48(1):35–44 e6. https://doi.org/10.1016/j.immuni.2017.11.013.
**a S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593(7860):607–11. https://doi.org/10.1038/s41586-021-03478-3.
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–43. https://doi.org/10.1038/s41586-019-1295-z.
Chai, Q., Yu, S., Zhong, Y., Lu, Z., Qiu, C., Yu, Y. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science. 2022;378(6616):eabq0132. https://doi.org/10.1126/science.abq0132.
Hochheiser IV, Pilsl M, Hagelueken G, Moecking J, Marleaux M, Brinkschulte R, et al. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature. 2022;604(7904):184–9. https://doi.org/10.1038/s41586-022-04467-w.
Zhao, N., Li, C.C., Di, B. & Xu, L.L. Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors. J Autoimmun. 2020;113102515. https://doi.org/10.1016/j.jaut.2020.102515.
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–53. https://doi.org/10.1016/j.immuni.2013.05.016.
Yasuda, K., Nakanishi, K. & Tsutsui, H. Interleukin-18 in Health and Disease. Int J Mol Sci. 2019;20(3). https://doi.org/10.3390/ijms20030649.
Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282(10):7416–23. https://doi.org/10.1074/jbc.M606721200.
Ranjan, K. & Pathak, C. FADD regulates NF-kappaB activation and promotes ubiquitination of cFLIPL to induce apoptosis. Sci Rep. 2016;622787. https://doi.org/10.1038/srep22787.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42. https://doi.org/10.1038/nature11986.
Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–59. https://doi.org/10.1038/cmi.2015.95.
Coll RC, Schroder K, Pelegrin P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653–68. https://doi.org/10.1016/j.tips.2022.04.003.
Di, A., **ong, S., Ye, Z., Malireddi, R.K.S., Kometani, S., Zhong, M. et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49(1):56–65 e4. https://doi.org/10.1016/j.immuni.2018.04.032.
Wang, Y., Zeng, Z., Ran, J., Peng, L., Wu, X., Ye, C. et al. The Critical Role of Potassium Efflux and Nek7 in Pasteurella multocida-Induced NLRP3 Inflammasome Activation. Front Microbiol. 2022;13849482. https://doi.org/10.3389/fmicb.2022.849482.
Liu, R., Liu, Y., Liu, C., Gao, A., Wang, L., Tang, H. et al. NEK7-Mediated Activation of NLRP3 Inflammasome Is Coordinated by Potassium Efflux/Syk/JNK Signaling During Staphylococcus aureus Infection. Front Immunol. 2021;12747370. https://doi.org/10.3389/fimmu.2021.747370.
Huang, L.S., Anas, M., Xu, J., Zhou, B., Toth, P.T., Krishnan, Y. et al. Endosomal trafficking of two-pore K(+) efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury. Elife. 2023;12. https://doi.org/10.7554/eLife.83842.
Samways, D.S., Li, Z. & Egan, T.M. Principles and properties of ion flow in P2X receptors. Front Cell Neurosci. 2014;86. https://doi.org/10.3389/fncel.2014.00006.
Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiol Rev. 2012;92(4):1577–617. https://doi.org/10.1152/physrev.00009.2012.
Dagvadorj, J., Mikulska-Ruminska, K., Tumurkhuu, G., Ratsimandresy, R.A., Carriere, J., Andres, A.M. et al. Recruitment of pro-IL-1alpha to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci U S A. 2021;118(1). https://doi.org/10.1073/pnas.2015632118.
Liu, H., Gu, C., Liu, M., Liu, G. & Wang, Y. NEK7 mediated assembly and activation of NLRP3 inflammasome downstream of potassium efflux in ventilator-induced lung injury. Biochem Pharmacol. 2020;177113998. https://doi.org/10.1016/j.bcp.2020.113998.
Green JP, Yu S, Martin-Sanchez F, Pelegrin P, Lopez-Castejon G, Lawrence CB, et al. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc Natl Acad Sci U S A. 2018;115(40):E9371–80. https://doi.org/10.1073/pnas.1812744115.
Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202. https://doi.org/10.1038/s41467-017-00227-x.
Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37(3):487–500. https://doi.org/10.1016/j.immuni.2012.06.013.
Domingo-Fernandez R, Coll RC, Kearney J, Breit S, O’Neill LAJ. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1beta transcription and activate the NLRP3 inflammasome. J Biol Chem. 2017;292(29):12077–87. https://doi.org/10.1074/jbc.M117.797126.
Mayes-Hopfinger L, Enache A, **e J, Huang CL, Kochl R, Tybulewicz VLJ, et al. Chloride sensing by WNK1 regulates NLRP3 inflammasome activation and pyroptosis. Nat Commun. 2021;12(1):4546. https://doi.org/10.1038/s41467-021-24784-4.
Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. https://doi.org/10.1016/j.cell.2007.11.028.
Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170(6):3029–36. https://doi.org/10.4049/jimmunol.170.6.3029.
Chu J, Thomas LM, Watkins SC, Franchi L, Nunez G, Salter RD. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86(5):1227–38. https://doi.org/10.1189/jlb.0309164.
Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 2015;194(8):3937–52. https://doi.org/10.4049/jimmunol.1402658.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. https://doi.org/10.1038/nature09663.
Barrera MJ, Aguilera S, Castro I, Carvajal P, Jara D, Molina C, et al. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjogren’s syndrome. Autoimmun Rev. 2021;20(8): 102867. https://doi.org/10.1016/j.autrev.2021.102867.
Li, C., Zhu, Y., Liu, W., Hayashi, T., **ang, W., He, S. et al. Increased mitochondrial fission induces NLRP3/cGAS-STING mediated pro-inflammatory pathways and apoptosis in UVB-irradiated immortalized human keratinocyte HaCaT cells. Arch Biochem Biophys. 2023;738109558. https://doi.org/10.1016/j.abb.2023.109558.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. https://doi.org/10.1016/j.immuni.2012.01.009.
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. https://doi.org/10.1038/ni.1980.
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560(7717):198–203. https://doi.org/10.1038/s41586-018-0372-z.
Sho T, Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol Appl Biochem. 2019;66(1):4–13. https://doi.org/10.1002/bab.1700.
Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191(8):4358–66. https://doi.org/10.4049/jimmunol.1301170.
Guan K, Wei C, Zheng Z, Song T, Wu F, Zhang Y, et al. MAVS Promotes Inflammasome Activation by Targeting ASC for K63-Linked Ubiquitination via the E3 Ligase TRAF3. J Immunol. 2015;194(10):4880–90. https://doi.org/10.4049/jimmunol.1402851.
Hsu CG, Li W, Sowden M, Chavez CL, Berk BC. Pnpt1 mediates NLRP3 inflammasome activation by MAVS and metabolic reprogramming in macrophages. Cell Mol Immunol. 2023;20(2):131–42. https://doi.org/10.1038/s41423-022-00962-2.
Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A. 2013;110(44):17963–8. https://doi.org/10.1073/pnas.1312571110.
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–23. https://doi.org/10.1016/j.immuni.2013.08.001.
Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, et al. Cutting Edge: Mitochondrial Assembly of the NLRP3 Inflammasome Complex Is Initiated at Priming. J Immunol. 2018;200(9):3047–52. https://doi.org/10.4049/jimmunol.1701723.
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–56. https://doi.org/10.1038/ni.1631.
Orlowski GM, Colbert JD, Sharma S, Bogyo M, Robertson SA, Rock KL. Multiple Cathepsins Promote Pro-IL-1beta Synthesis and NLRP3-Mediated IL-1beta Activation. J Immunol. 2015;195(4):1685–97. https://doi.org/10.4049/jimmunol.1500509.
Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem. 2014;289(13):9158–71. https://doi.org/10.1074/jbc.M113.531202.
Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE. 2013;8(1): e55375. https://doi.org/10.1371/journal.pone.0055375.
Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS ONE. 2009;4(8): e6510. https://doi.org/10.1371/journal.pone.0006510.
Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109(28):11282–7. https://doi.org/10.1073/pnas.1117765109.
Katsnelson MA, Lozada-Soto KM, Russo HM, Miller BA, Dubyak GR. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. Am J Physiol Cell Physiol. 2016;311(1):C83–100. https://doi.org/10.1152/ajpcell.00298.2015.
Brubaker, S.W., Brewer, S.M., Massis, L.M., Napier, B.A. & Monack, D.M. A Rapid Caspase-11 Response Induced by IFNgamma Priming Is Independent of Guanylate Binding Proteins. iScience. 2020;23(10):101612. https://doi.org/10.1016/j.isci.2020.101612.
Balakrishnan, A., Karki, R., Berwin, B., Yamamoto, M. & Kanneganti, T.D. Guanylate binding proteins facilitate caspase-11-dependent pyroptosis in response to type 3 secretion system-negative Pseudomonas aeruginosa. Cell Death Discov. 2018;43. https://doi.org/10.1038/s41420-018-0068-z.
Gaidt, M.M. & Hornung, V. Alternative inflammasome activation enables IL-1beta release from living cells. Curr Opin Immunol. 2017;447–13. https://doi.org/10.1016/j.coi.2016.10.007.
Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44(4):833–46. https://doi.org/10.1016/j.immuni.2016.01.012.
Chan, A.H. & Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med. 2020;217(1). https://doi.org/10.1084/jem.20190314.
Netea, M.G., van de Veerdonk, F.L., van der Meer, J.W., Dinarello, C.A. & Joosten, L.A. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;3349–77. https://doi.org/10.1146/annurev-immunol-032414-112306.
Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, et al. Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase. J Biol Chem. 2015;290(29):18124–33. https://doi.org/10.1074/jbc.M115.645549.
Song, H., Liu, B., Huai, W., Yu, Z., Wang, W., Zhao, J. et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016;713727. https://doi.org/10.1038/ncomms13727.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1–2):62–73. https://doi.org/10.1016/j.cell.2014.11.047.
Song, N., Liu, Z.S., Xue, W., Bai, Z.F., Wang, Q.Y., Dai, J. et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68(1):185–197 e6. https://doi.org/10.1016/j.molcel.2017.08.017.
Zhang Z, Meszaros G, He WT, Xu Y, de Fatima Magliarelli H, Mailly L, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214(9):2671–93. https://doi.org/10.1084/jem.20162040.
Stutz A, Kolbe CC, Stahl R, Horvath GL, Franklin BS, van Ray O, et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med. 2017;214(6):1725–36. https://doi.org/10.1084/jem.20160933.
Akther, M., Haque, M.E., Park, J., Kang, T.B. & Lee, K.H. NLRP3 Ubiquitination-A New Approach to Target NLRP3 Inflammasome Activation. Int J Mol Sci. 2021;22(16). https://doi.org/10.3390/ijms22168780.
Barry R, John SW, Liccardi G, Tenev T, Jaco I, Chen CH, et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat Commun. 2018;9(1):3001. https://doi.org/10.1038/s41467-018-05321-2.
Leu, S.Y., Tsang, Y.L., Ho, L.C., Yang, C.C., Shao, A.N., Chang, C.Y. et al. NLRP3 inflammasome activation, metabolic danger signals, and protein binding partners. J Endocrinol. 2023;257(2). https://doi.org/10.1530/JOE-22-0184.
Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336(6080):481–5. https://doi.org/10.1126/science.1217141.
Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16(5):476–84. https://doi.org/10.1038/ni.3119.
Man SM, Place DE, Kuriakose T, Kanneganti TD. Interferon-inducible guanylate-binding proteins at the interface of cell-autonomous immunity and inflammasome activation. J Leukoc Biol. 2017;101(1):143–50. https://doi.org/10.1189/jlb.4MR0516-223R.
Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–4. https://doi.org/10.1038/nature11290.
Yoshida K, Okamura H, Hiroshima Y, Abe K, Kido JI, Shinohara Y, et al. PKR induces the expression of NLRP3 by regulating the NF-kappaB pathway in Porphyromonas gingivalis-infected osteoblasts. Exp Cell Res. 2017;354(1):57–64. https://doi.org/10.1016/j.yexcr.2017.03.028.
He Y, Franchi L, Nunez G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur J Immunol. 2013;43(5):1147–52. https://doi.org/10.1002/eji.201243187.
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–5. https://doi.org/10.1038/ng756.
Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76(6):942–7. https://doi.org/10.1136/annrheumdis-2016-209686.
Mortimer L, Moreau F, MacDonald JA, Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17(10):1176–86. https://doi.org/10.1038/ni.3538.
Saresella, M., La Rosa, F., Piancone, F., Zoppis, M., Marventano, I., Calabrese, E. et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer's disease. Mol Neurodegener. 2016;1123. https://doi.org/10.1186/s13024-016-0088-1.
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65. https://doi.org/10.1038/ni.1636.
Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzumura A. Oligomeric amyloid beta induces IL-1beta processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis. 2013;4(12): e975. https://doi.org/10.1038/cddis.2013.503.
Italiani P, Puxeddu I, Napoletano S, Scala E, Melillo D, Manocchio S, et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation. 2018;15(1):342. https://doi.org/10.1186/s12974-018-1376-1.
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1beta, IL-6, TNF- alpha and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci Rep. 2018;8(1):12050. https://doi.org/10.1038/s41598-018-30487-6.
Dempsey, C., Rubio Araiz, A., Bryson, K.J., Finucane, O., Larkin, C., Mills, E.L. et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61306–316. https://doi.org/10.1016/j.bbi.2016.12.014.
Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S, et al. NLRP3 Inflammasome Inhibitor Ameliorates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2018;55(3):1977–87. https://doi.org/10.1007/s12035-017-0467-9.
Wallisch JS, Simon DW, Bayir H, Bell MJ, Kochanek PM, Clark RSB. Cerebrospinal Fluid NLRP3 is Increased After Severe Traumatic Brain Injury in Infants and Children. Neurocrit Care. 2017;27(1):44–50. https://doi.org/10.1007/s12028-017-0378-7.
Ramos-Cejudo, J., Wisniewski, T., Marmar, C., Zetterberg, H., Blennow, K., de Leon, M.J. et al. Traumatic Brain Injury and Alzheimer's Disease: The Cerebrovascular Link. EBioMedicine. 2018;2821–30. https://doi.org/10.1016/j.ebiom.2018.01.021.
Delic V, Beck KD, Pang KCH, Citron BA. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol Commun. 2020;8(1):45. https://doi.org/10.1186/s40478-020-00924-7.
Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(Pt B):75–80. https://doi.org/10.1016/j.mcn.2015.03.001.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. https://doi.org/10.1038/nature04516.
Braga, T.T., Forni, M.F., Correa-Costa, M., Ramos, R.N., Barbuto, J.A., Branco, P. et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci Rep. 2017;739884. https://doi.org/10.1038/srep39884.
Wu, X.Y., Li, K.T., Yang, H.X., Yang, B., Lu, X., Zhao, L.D. et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J Autoimmun. 2020;106102336. https://doi.org/10.1016/j.jaut.2019.102336.
Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–57. https://doi.org/10.1016/j.cell.2011.04.022.
Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530(1–3):73–8. https://doi.org/10.1016/s0014-5793(02)03416-6.
Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, et al. Nlrp6 regulates intestinal antiviral innate immunity. Science. 2015;350(6262):826–30. https://doi.org/10.1126/science.aab3145.
Levy M, Shapiro H, Thaiss CA, Elinav E. NLRP6: A Multifaceted Innate Immune Sensor. Trends Immunol. 2017;38(4):248–60. https://doi.org/10.1016/j.it.2017.01.001.
Ghimire L, Paudel S, ** L, Baral P, Cai S, Jeyaseelan S. NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function. PLoS Pathog. 2018;14(9): e1007308. https://doi.org/10.1371/journal.ppat.1007308.
Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488(7411):389–93. https://doi.org/10.1038/nature11250.
Ydens, E., Demon, D., Lornet, G., De Winter, V., Timmerman, V., Lamkanfi, M. et al. Nlrp6 promotes recovery after peripheral nerve injury independently of inflammasomes. J Neuroinflammation. 2015;12143. https://doi.org/10.1186/s12974-015-0367-8.
Hara, H., Seregin, S.S., Yang, D., Fukase, K., Chamaillard, M., Alnemri, E.S. et al. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell. 2018;175(6):1651–1664 e14. https://doi.org/10.1016/j.cell.2018.09.047.
Radulovic K, Ayata CK, Mak’Anyengo R, Lechner K, Wuggenig P, Kaya B, et al. NLRP6 Deficiency in CD4 T Cells Decreases T Cell Survival Associated with Increased Cell Death. J Immunol. 2019;203(2):544–56. https://doi.org/10.4049/jimmunol.1800938.
Birchenough GM, Nystrom EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352(6293):1535–42. https://doi.org/10.1126/science.aaf7419.
Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163(6):1428–43. https://doi.org/10.1016/j.cell.2015.10.048.
Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–22. https://doi.org/10.1016/j.cell.2014.04.007.
Zheng D, Kern L, Elinav E. The NLRP6 inflammasome. Immunology. 2021;162(3):281–9. https://doi.org/10.1111/imm.13293.
Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol. 2010;22(9):717–28. https://doi.org/10.1093/intimm/dxq058.
Liu W, Liu J, Wang W, Wang Y, Ouyang X. NLRP6 Induces Pyroptosis by Activation of Caspase-1 in Gingival Fibroblasts. J Dent Res. 2018;97(12):1391–8. https://doi.org/10.1177/0022034518775036.
Lu WL, Zhang L, Song DZ, Yi XW, Xu WZ, Ye L, et al. NLRP6 suppresses the inflammatory response of human periodontal ligament cells by inhibiting NF-kappaB and ERK signal pathways. Int Endod J. 2019;52(7):999–1009. https://doi.org/10.1111/iej.13091.
Bracey, N.A., Platnich, J.M., Lau, A., Chung, H., Hyndman, M.E., MacDonald, J.A. et al. Tissue-selective alternate promoters guide NLRP6 expression. Life Sci Alliance. 2021;4(3). https://doi.org/10.26508/lsa.202000897.
Angosto-Bazarra D, Molina-Lopez C, Pelegrin P. Physiological and pathophysiological functions of NLRP6: pro- and anti-inflammatory roles. Commun Biol. 2022;5(1):524. https://doi.org/10.1038/s42003-022-03491-w.
Shen C, Lu A, **e WJ, Ruan J, Negro R, Egelman EH, et al. Molecular mechanism for NLRP6 inflammasome assembly and activation. Proc Natl Acad Sci U S A. 2019;116(6):2052–7. https://doi.org/10.1073/pnas.1817221116.
Janani C, Ranjitha Kumari BDPPAR. gamma gene–a review. Diabetes Metab Syndr. 2015;9(1):46–50. https://doi.org/10.1016/j.dsx.2014.09.015.
Kempster SL, Belteki G, Forhead AJ, Fowden AL, Catalano RD, Lam BY, et al. Developmental control of the Nlrp6 inflammasome and a substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G253–63. https://doi.org/10.1152/ajpgi.00397.2010.
Wang G, Wang Q, Bai J, Zhao N, Wang Y, Zhou R, et al. Upregulation of Intestinal NLRP6 Inflammasomes After Roux-en-Y Gastric Bypass Promotes Gut Immune Homeostasis. Obes Surg. 2020;30(1):327–35. https://doi.org/10.1007/s11695-019-04152-4.
Shen, C., Li, R., Negro, R., Cheng, J., Vora, S.M., Fu, T.M. et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell. 2021;184(23):5759–5774 e20. https://doi.org/10.1016/j.cell.2021.09.032.
Ghimire L, Paudel S, ** L, Jeyaseelan S. The NLRP6 inflammasome in health and disease. Mucosal Immunol. 2020;13(3):388–98. https://doi.org/10.1038/s41385-020-0256-z.
Leng F, Yin H, Qin S, Zhang K, Guan Y, Fang R, et al. NLRP6 self-assembles into a linear molecular platform following LPS binding and ATP stimulation. Sci Rep. 2020;10(1):198. https://doi.org/10.1038/s41598-019-57043-0.
Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341(6142):172–5. https://doi.org/10.1126/science.1236381.
Mukherjee S, Kumar R, Tsakem Lenou E, Basrur V, Kontoyiannis DL, Ioakeimidis F, et al. Deubiquitination of NLRP6 inflammasome by Cyld critically regulates intestinal inflammation. Nat Immunol. 2020;21(6):626–35. https://doi.org/10.1038/s41590-020-0681-x.
Nie, H., Hu, Y., Guo, W., Wang, W., Yang, Q., Dong, Q. et al. miR-331–3p Inhibits Inflammatory Response after Intracerebral Hemorrhage by Directly Targeting NLRP6. Biomed Res Int. 2020;20206182464. https://doi.org/10.1155/2020/6182464.
Xu X, Zhu X, Wang C, Li Y, Fan C, Kao X. microRNA-650 promotes inflammation induced apoptosis of intestinal epithelioid cells by targeting NLRP6. Biochem Biophys Res Commun. 2019;517(4):551–6. https://doi.org/10.1016/j.bbrc.2019.06.077.
Glorioso N, Herrera VL, Didishvili T, Ortu MF, Zaninello R, Fresu G, et al. Sex-specific effects of NLRP6/AVR and ADM loci on susceptibility to essential hypertension in a Sardinian population. PLoS ONE. 2013;8(10): e77562. https://doi.org/10.1371/journal.pone.0077562.
Li G, Tian X, Lv D, Zhang L, Zhang Z, Wang J, et al. NLRP7 is expressed in the ovine ovary and associated with in vitro pre-implantation embryo development. Reproduction. 2019;158(5):415–27. https://doi.org/10.1530/REP-19-0081.
Radian AD, de Almeida L, Dorfleutner A, Stehlik C. NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease. Microbes Infect. 2013;15(8–9):630–9. https://doi.org/10.1016/j.micinf.2013.04.001.
Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity. 2012;36(3):464–76. https://doi.org/10.1016/j.immuni.2012.02.001.
Wang CM, Dixon PH, Decordova S, Hodges MD, Sebire NJ, Ozalp S, et al. Identification of 13 novel NLRP7 mutations in 20 families with recurrent hydatidiform mole; missense mutations cluster in the leucine-rich region. J Med Genet. 2009;46(8):569–75. https://doi.org/10.1136/jmg.2008.064196.
Messaed C, Chebaro W, Di Roberto RB, Rittore C, Cheung A, Arseneau J, et al. NLRP7 in the spectrum of reproductive wastage: rare non-synonymous variants confer genetic susceptibility to recurrent reproductive wastage. J Med Genet. 2011;48(8):540–8. https://doi.org/10.1136/jmg.2011.089144.
Zheng, D., Liwinski, T. & Elinav, E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;636. https://doi.org/10.1038/s41421-020-0167-x.
Radian, A.D., Khare, S., Chu, L.H., Dorfleutner, A. & Stehlik, C. ATP binding by NLRP7 is required for inflammasome activation in response to bacterial lipopeptides. Mol Immunol. 2015;67(2 Pt B):294–302. https://doi.org/10.1016/j.molimm.2015.06.013.
Murdoch S, Djuric U, Mazhar B, Seoud M, Khan R, Kuick R, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006;38(3):300–2. https://doi.org/10.1038/ng1740.
Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61(7):1113–21. https://doi.org/10.1002/glia.22499.
Rossi, M.N., Pascarella, A., Licursi, V., Caiello, I., Taranta, A., Rega, L.R. et al. NLRP2 Regulates Proinflammatory and Antiapoptotic Responses in Proximal Tubular Epithelial Cells. Front Cell Dev Biol. 2019;7252. https://doi.org/10.3389/fcell.2019.00252.
Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J Biol Chem. 2004;279(50):51897–907. https://doi.org/10.1074/jbc.M406741200.
Razmara M, Srinivasula SM, Wang L, Poyet JL, Geddes BJ, DiStefano PS, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277(16):13952–8. https://doi.org/10.1074/jbc.M107811200.
Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit Rev Immunol. 2010;30(5):463–87. https://doi.org/10.1615/critrevimmunol.v30.i5.50.
Zhang, Q., Sun, Y., He, Z., Xu, Y., Li, X., Ding, J. et al. Kynurenine regulates NLRP2 inflammasome in astrocytes and its implications in depression. Brain Behav Immun. 2020;88471–481. https://doi.org/10.1016/j.bbi.2020.04.016.
Nyul-Toth, A., Kozma, M., Nagyoszi, P., Nagy, K., Fazakas, C., Hasko, J. et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav Immun. 2017;64220–231. https://doi.org/10.1016/j.bbi.2017.04.010.
Kufer TA, Sansonetti PJ. NLR functions beyond pathogen recognition. Nat Immunol. 2011;12(2):121–8. https://doi.org/10.1038/ni.1985.
Amoushahi M, Steffensen LL, Galieva A, Agger J, Heuck A, Siupka P, et al. Maternally contributed Nlrp9b expressed in human and mouse ovarian follicles contributes to early murine preimplantation development. J Assist Reprod Genet. 2020;37(6):1355–65. https://doi.org/10.1007/s10815-020-01767-w.
Kanzaki S, Tamura S, Ito T, Wakabayashi M, Saito K, Kato S, et al. Involvement of Nlrp9a/b/c in mouse preimplantation development. Reproduction. 2020;160(2):181–91. https://doi.org/10.1530/REP-19-0516.
Peng H, Lin X, Liu F, Wang C, Zhang W. NLRP9B protein is dispensable for oocyte maturation and early embryonic development in the mouse. J Reprod Dev. 2015;61(6):559–64. https://doi.org/10.1262/jrd.2015-050.
Wei Y, Li L, Zhou X, Zhang QY, Dunbar A, Liu F, et al. Generation and characterization of a novel Cyp2a(4/5)bgs-null mouse model. Drug Metab Dispos. 2013;41(1):132–40. https://doi.org/10.1124/dmd.112.048736.
Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546(7660):667–70. https://doi.org/10.1038/nature22967.
Ngo C, Man SM. NLRP9b: a novel RNA-sensing inflammasome complex. Cell Res. 2017;27(11):1302–3. https://doi.org/10.1038/cr.2017.93.
Yanling Q, **aoning C, Fei B, Liyun F, Huizhong H, Daqing S. Inhibition of NLRP9b attenuates acute lung injury through suppressing inflammation, apoptosis and oxidative stress in murine and cell models. Biochem Biophys Res Commun. 2018;503(2):436–43. https://doi.org/10.1016/j.bbrc.2018.04.079.
Mullins B, Chen J. NLRP9 in innate immunity and inflammation. Immunology. 2021;162(3):262–7. https://doi.org/10.1111/imm.13290.
Tadaki, H., Saitsu, H., Nishimura-Tadaki, A., Imagawa, T., Kikuchi, M., Hara, R. et al. De novo 19q13.42 duplications involving NLRP gene cluster in a patient with systemic-onset juvenile idiopathic arthritis. J Hum Genet. 2011;56(5):343–7. https://doi.org/10.1038/jhg.2011.16.
Gil-Varea E, Urcelay E, Vilarino-Guell C, Costa C, Midaglia L, Matesanz F, et al. Exome sequencing study in patients with multiple sclerosis reveals variants associated with disease course. J Neuroinflammation. 2018;15(1):265. https://doi.org/10.1186/s12974-018-1307-1.
Fernandez, M.V., Budde, J., Del-Aguila, J.L., Ibanez, L., Deming, Y., Harari, O. et al. Evaluation of Gene-Based Family-Based Methods to Detect Novel Genes Associated With Familial Late Onset Alzheimer Disease. Front Neurosci. 2018;12209. https://doi.org/10.3389/fnins.2018.00209.
Poli, G., Brancorsini, S., Cochetti, G., Barillaro, F., Egidi, M.G. & Mearini, E. Expression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNA. Urol Oncol. 2015;33(12):505 e1–7. https://doi.org/10.1016/j.urolonc.2015.07.012.
Castano-Rodriguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: a case-control study and gene expression analyses. PLoS ONE. 2014;9(6): e98899. https://doi.org/10.1371/journal.pone.0098899.
Wang Y, Hasegawa M, Imamura R, Kinoshita T, Kondo C, Konaka K, et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int Immunol. 2004;16(6):777–86. https://doi.org/10.1093/intimm/dxh081.
Damm A, Lautz K, Kufer TA. Roles of NLRP10 in innate and adaptive immunity. Microbes Infect. 2013;15(6–7):516–23. https://doi.org/10.1016/j.micinf.2013.03.008.
Imamura R, Wang Y, Kinoshita T, Suzuki M, Noda T, Sagara J, et al. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J Immunol. 2010;184(10):5874–84. https://doi.org/10.4049/jimmunol.0900779.
Prochnicki T, Vasconcelos MB, Robinson KS, Mangan MSJ, De Graaf D, Shkarina K, et al. Mitochondrial damage activates the NLRP10 inflammasome. Nat Immunol. 2023;24(4):595–603. https://doi.org/10.1038/s41590-023-01451-y.
Zheng D, Mohapatra G, Kern L, He Y, Shmueli MD, Valdes-Mas R, et al. Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation. Nat Immunol. 2023;24(4):585–94. https://doi.org/10.1038/s41590-023-01450-z.
Vacca, M., Bohme, J., Zambetti, L.P., Khameneh, H.J., Paleja, B.S., Laudisi, F. et al. NLRP10 Enhances CD4(+) T-Cell-Mediated IFNgamma Response via Regulation of Dendritic Cell-Derived IL-12 Release. Front Immunol. 2017;81462. https://doi.org/10.3389/fimmu.2017.01462.
Masters SLT. things to know about NLRP10. Nat Immunol. 2023;24(4):561–2. https://doi.org/10.1038/s41590-023-01466-5.
Williams KL, Taxman DJ, Linhoff MW, Reed W, Ting JP. Cutting edge: Monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J Immunol. 2003;170(11):5354–8. https://doi.org/10.4049/jimmunol.170.11.5354.
Fiorentino L, Stehlik C, Oliveira V, Ariza ME, Godzik A, Reed JC. A novel PAAD-containing protein that modulates NF-kappa B induction by cytokines tumor necrosis factor-alpha and interleukin-1beta. J Biol Chem. 2002;277(38):35333–40. https://doi.org/10.1074/jbc.M200446200.
Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36(5):742–54. https://doi.org/10.1016/j.immuni.2012.03.012.
Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280(48):39914–24. https://doi.org/10.1074/jbc.M502820200.
Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, et al. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178(3):1256–60. https://doi.org/10.4049/jimmunol.178.3.1256.
Bulte, D., Rigamonti, C., Romano, A. & Mortellaro, A. Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells. 2023;12(13). https://doi.org/10.3390/cells12131766.
Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37(1):96–107. https://doi.org/10.1016/j.immuni.2012.07.006.
Tuladhar, S. & Kanneganti, T.D. NLRP12 in innate immunity and inflammation. Mol Aspects Med. 2020;76100887. https://doi.org/10.1016/j.mam.2020.100887.
Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105(5):1614–9. https://doi.org/10.1073/pnas.0708616105.
Wang HF. NLRP12-associated systemic autoinflammatory diseases in children. Pediatr Rheumatol Online J. 2022;20(1):9. https://doi.org/10.1186/s12969-022-00669-8.
Vitale, A., Rigante, D., Maggio, M.C., Emmi, G., Romano, M., Silvestri, E. et al. Rare NLRP12 variants associated with the NLRP12-autoinflammatory disorder phenotype: an Italian case series. Clin Exp Rheumatol. 2013;31(3 Suppl 77):155–6. https://www.ncbi.nlm.nih.gov/pubmed/24064030
Jeru I, Hentgen V, Normand S, Duquesnoy P, Cochet E, Delwail A, et al. Role of interleukin-1beta in NLRP12-associated autoinflammatory disorders and resistance to anti-interleukin-1 therapy. Arthritis Rheum. 2011;63(7):2142–8. https://doi.org/10.1002/art.30378.
Placha, D. & Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics. 2021;13(1). https://doi.org/10.3390/pharmaceutics13010064.
Lahooti, B., Chhibber, T., Bagchi, S., Varahachalam, S.P. & Jayant, R.D. Therapeutic role of inflammasome inhibitors in neurodegenerative disorders. Brain Behav Immun. 2021;91771–783. https://doi.org/10.1016/j.bbi.2020.11.004.
Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123(11):4695–705. https://doi.org/10.1172/JCI71543.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. https://doi.org/10.1038/ni.1935.
Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187(1):61–70. https://doi.org/10.1083/jcb.200903124.
Zahid, A., Li, B., Kombe, A.J.K., **, T. & Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;102538. https://doi.org/10.3389/fimmu.2019.02538.
Oronsky B, Reid TR, Larson C, Caroen S, Quinn M, Burbano E, et al. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 2019;15(30):3427–33. https://doi.org/10.2217/fon-2019-0317.
Jayabalan N, Oronsky B, Cabrales P, Reid T, Caroen S, Johnson AM, et al. A Review of RRx-001: A Late-Stage Multi-Indication Inhibitor of NLRP3 Activation and Chronic Inflammation. Drugs. 2023;83(5):389–402. https://doi.org/10.1007/s40265-023-01838-z.
Parmar DV, Kansagra KA, Momin T, Patel HB, Jansari GA, Bhavsar J, et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Oral NLRP3 Inflammasome Inhibitor ZYIL1: First-in-Human Phase 1 Studies (Single Ascending Dose and Multiple Ascending Dose). Clin Pharmacol Drug Dev. 2023;12(2):202–11. https://doi.org/10.1002/cpdd.1162.
Liu W, Guo W, Wu J, Luo Q, Tao F, Gu Y, et al. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem Pharmacol. 2013;85(10):1504–12. https://doi.org/10.1016/j.bcp.2013.03.008.
Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346(6212):1000–3. https://doi.org/10.1126/science.1261754.
Chen, Q.-L., Yin, H.-R., He, Q.-Y. & Wang, Y. Targeting the NLRP3 inflammasome as new therapeutic avenue for inflammatory bowel disease. Biomedicine & Pharmacotherapy. 2021;138. https://doi.org/10.1016/j.biopha.2021.111442.
He Y, Varadarajan S, Munoz-Planillo R, Burberry A, Nakamura Y, Nunez G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289(2):1142–50. https://doi.org/10.1074/jbc.M113.515080.
Zheng, J., Jiang, Z., Song, Y., Huang, S., Du, Y., Yang, X. et al. 3,4-Methylenedioxy-beta-Nitrostyrene Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis by Inhibiting the NLRP3 Inflammasome. Front Pharmacol. 2022;13866228. https://doi.org/10.3389/fphar.2022.866228.
Han, S., He, Z., Hu, X., Li, X., Zheng, K., Huang, Y. et al. Inhibiting NLRP3 Inflammasome Activation by CY-09 Helps to Restore Cerebral Glucose Metabolism in 3xTg-AD Mice. Antioxidants (Basel). 2023;12(3). https://doi.org/10.3390/antiox12030722.
Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–14. https://doi.org/10.1038/nrcardio.2017.161.
He, H., Jiang, H., Chen, Y., Ye, J., Wang, A., Wang, C. et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. 2018;9(1):2550.
Klughammer B, Piali L, Nica A, Nagel S, Bailey L, Jochum C, et al. A randomized, double-blind phase 1b study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of the NLRP3 inhibitor selnoflast in patients with moderate to severe active ulcerative colitis. Clin Transl Med. 2023;13(11): e1471. https://doi.org/10.1002/ctm2.1471.
Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS ONE. 2011;6(12): e29539. https://doi.org/10.1371/journal.pone.0029539.
Isakov E, Weisman-Shomer P, Benhar M. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim Biophys Acta. 2014;1840(10):3153–61. https://doi.org/10.1016/j.bbagen.2014.07.012.
Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, Tsuneyama K, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 2014;96(6):1087–100. https://doi.org/10.1189/jlb.3A0114-005RR.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9. https://doi.org/10.1038/nm.3804.
Jesus, A.A. & Goldbach-Mansky, R. IL-1 blockade in autoinflammatory syndromes. Annu Rev Med. 2014;65223–44. https://doi.org/10.1146/annurev-med-061512-150641.
Uchiyama, A., Kosaka, K., Okada, K. & Motegi, S.i. Possible suppressive effects of tranilast on NLRP3 inflammasome activation in necrobiosis lipoidica. The Journal of Dermatology. 2022;49(12). https://doi.org/10.1111/1346-8138.16521.
Dagher D, Elmansi H, Nasr JJ, El-Enany N. Utility of a novel turn-off fluorescence probe for the determination of tranilast, an adjunctive drug for patients with severe COVID-19. RSC Adv. 2022;12(34):22044–53. https://doi.org/10.1039/d2ra02239g.
Mao L, Kitani A, Hiejima E, Montgomery-Recht K, Zhou W, Fuss I, et al. Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1β–mediated colitis. J Clin Investig. 2020;130(4):1793–807. https://doi.org/10.1172/jci128322.
Zhao Y, Guo Q, Zhu Q, Tan R, Bai D, Bu X, et al. Flavonoid VI-16 protects against DSS-induced colitis by inhibiting Txnip-dependent NLRP3 inflammasome activation in macrophages via reducing oxidative stress. Mucosal Immunol. 2019;12(5):1150–63. https://doi.org/10.1038/s41385-019-0177-x.
Pellegrini, C., Fornai, M., Colucci, R., Benvenuti, L., D'Antongiovanni, V., Natale, G. et al. A Comparative Study on the Efficacy of NLRP3 Inflammasome Signaling Inhibitors in a Pre-clinical Model of Bowel Inflammation. Front Pharmacol. 2018;91405. https://doi.org/10.3389/fphar.2018.01405.
Zhang, X., Wang, Z., Zheng, Y., Yu, Q., Zeng, M., Bai, L. et al. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int J Mol Med. 2023;51(4). https://doi.org/10.3892/ijmm.2023.5238.
Hu JJ, Liu X, **a S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–45. https://doi.org/10.1038/s41590-020-0669-6.
Harrison D, Billinton A, Bock MG, Doedens JR, Gabel CA, Holloway MK, et al. Discovery of Clinical Candidate NT-0796, a Brain-Penetrant and Highly Potent NLRP3 Inflammasome Inhibitor for Neuroinflammatory Disorders. J Med Chem. 2023;66(21):14897–911. https://doi.org/10.1021/acs.jmedchem.3c01398.
Rabinovich E, Fromowitz A, Ajibade O, Sahu S, Verma D, Aminov S, et al. The Dual Inflammasome/Myddosome Inhibitor HT-6184 Restores Erythropoiesis in MDS/AML. Blood. 2023;142(Supplement 1):1417–1417. https://doi.org/10.1182/blood-2023-182289.
**, X., Liu, D., Zhou, X., Luo, X., Huang, Q. & Huang, Y. Entrectinib inhibits NLRP3 inflammasome and inflammatory diseases by directly targeting NEK7. Cell Reports Medicine. 2023;4(12). https://doi.org/10.1016/j.xcrm.2023.101310.
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285(13):9792–802. https://doi.org/10.1074/jbc.M109.082305.
Maier NK, Leppla SH, Moayeri M. The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J Immunol. 2015;194(6):2776–85. https://doi.org/10.4049/jimmunol.1401611.
Qin Q, Liu H, Shou J, Jiang Y, Yu H, Wang X. The inhibitor effect of RKIP on inflammasome activation and inflammasome-dependent diseases. Cell Mol Immunol. 2021;18(4):992–1004. https://doi.org/10.1038/s41423-020-00525-3.
Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4(9): e790. https://doi.org/10.1038/cddis.2013.326.
Docherty CA, Fernando AJ, Rosli S, Lam M, Dolle RE, Navia MA, et al. A novel dual NLRP1 and NLRP3 inflammasome inhibitor for the treatment of inflammatory diseases. Clin Transl Immunology. 2023;12(6): e1455. https://doi.org/10.1002/cti2.1455.
Maier NK, Crown D, Liu J, Leppla SH, Moayeri M. Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes. J Immunol. 2014;192(2):763–70. https://doi.org/10.4049/jimmunol.1301434.
Harris PA, Duraiswami C, Fisher DT, Fornwald J, Hoffman SJ, Hofmann G, et al. High throughput screening identifies ATP-competitive inhibitors of the NLRP1 inflammasome. Bioorg Med Chem Lett. 2015;25(14):2739–43. https://doi.org/10.1016/j.bmcl.2015.05.032.
Mao L, Kitani A, Similuk M, Oler AJ, Albenberg L, Kelsen J, et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J Clin Invest. 2018;128(5):1793–806. https://doi.org/10.1172/JCI98642.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grants No. 2022YFC2304102), the National Natural Science Foundation of China (Grant No. 31971129 and 82272301), the Anhui Provincial Key Research and Development Program (2022e07020013), the Fundamental Research Funds for the Central Universities (WK9100000001).
Funding
This work was funded by the National Key Research and Development Program of China (Grants No. 2022YFC2304102), the National Natural Science Foundation of China (Grant No. 31971129 and 82272301), the Anhui Provincial Key Research and Development Program (2022e07020013), the Fundamental Research Funds for the Central Universities (WK9100000001).
Author information
Authors and Affiliations
Contributions
ZX initiated and conceptualized and formulated the main idea, designed the study, and wrote the first draft of the paper. JAKK affined and improved the overall research idea, extensively reviewed the first draft and edited the final version of the manuscript. SD, HZ, and SW provided substantial ideas and help in manuscript writing. JR, YZ and TJ supervised, provided helpful discussion, reviewed, and approved the final version of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Z., Kombe Kombe, A.J., Deng, S. et al. NLRP inflammasomes in health and disease. Mol Biomed 5, 14 (2024). https://doi.org/10.1186/s43556-024-00179-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43556-024-00179-x