Abstract
Biochar is considered to be a possible means of carbon sequestration to alleviate climate change. However, the dynamics of the microbial community during wood decomposition after biochar application remain poorly understood. In this study, the wood-inhabiting bacterial community composition and its potential functions during a two-year decomposition period after the addition of different amounts of biochar (0.5 kg m−2 and 1.0 kg m−2), and at different biochar pyrolysis temperatures (500 °C and 650 °C), in a boreal Scots pine forest, were analyzed using Illumina NovaSeq sequencing combined with Functional Annotation of Prokaryotic Taxa (FAPROTAX). The results showed that the wood decomposition rates increased after biochar addition to the soil surface in the second year. Treatment with biochar produced at high temperatures increased the diversity of wood-inhabiting bacteria more than that produced at low temperatures (P < 0.05). The wood-inhabiting bacterial diversity and species richness decreased with decomposition time. The biochar treatments changed the wood-inhabiting bacterial community structure during the decomposition period. The pyrolysis temperature and the amount of applied biochar had no effect on the bacterial community structure but shifted the abundance of certain bacterial taxa. Similarly, biochar application shifted the wood-inhabiting bacterial community function in the first year, but not in the second year. The wood-inhabiting bacterial community and function were affected by soil pH, soil water content, and soil total nitrogen. The results provide useful information on biochar application for future forest management practices. Long-term monitoring is needed to better understand the effects of biochar application on nutrient cycling in boreal forests.
Similar content being viewed by others
Introduction
Biochar is carbon-rich, highly aromatic, and stable solid material made of crop waste, wood, and other biological materials under anaerobic conditions and at high pyrolysis temperatures (usually < 700 °C) [1, 2] It is an effective material to boost soil fertility, and it may also be effective for carbon sequestration to mitigate climate change in forest ecological systems [3, 4]. Biochar application can increase carbon storage, the C/N ratio, and water content in boreal forest soils [5], and in general can increase the net carbon input of northern forests [6]. Soil nitrogen exists in organic form in boreal forests, and the low nitrogen mineralization rate limits tree growth [7]. Our previous study from the same study area showed that the application of biochar in boreal forests can also increase the net nitrogen mineralization and nitrification rates and increase tree growth [8].
Organic matter decomposition is one of the key processes of nutrient cycling in forest ecosystems [9]. The litter decomposition is directly linked to microbial activities, which alter the chemical compounds of litter and regulate the dynamics of soil carbon and nitrogen [10]. The microbial community in litter might be influenced not only by the litter type, but also by the properties of the surrounding soil, vegetation, and soil microorganisms [11]. Bacteria make up the majority of soil microorganisms, accounting for over 80%, and are vulnerable to variations in pH and the availability of C sources [12]. They are one of the earliest organisms to colonize dead wood and metabolize easily degradable and available substrates [13], which can affect the structure of wood during degradation [14]. In the initial stage of wood decay, bacteria likely undergo a succession before the fungal occupation of the microbial community [15]. It has been found that changes in the soil bacterial community composition can significantly impact the wood-associated bacterial structure [16]. A previous study showed that the bacterial community undergoes a series of colonizations in the course of the decomposition of wood, and Proteobacteria, Actinomycetes, and Bacteroides are the most abundant taxa [17].
Biochar application in a forest can both directly and indirectly affect the physical and chemical properties of forest soil, which changes the soil microbial abundance, composition, and function [18]. It has been shown that biochar application can increase soil microbial biomass and significantly shift soil microbial community composition due to the special properties of biochar [19]. The properties of biochar depend on the production method, pyrolysis temperature, and raw material type [20] It has been reported that biochar produced at relatively high temperatures (600–700 °C) has a high proportion of aromatic C, large porosity, and a low cation exchange capacity [31]. PCR was carried out in 50 μL reaction mixtures with the following components: 25 µL 2 × Premix Taq, 1 µL Forward Primer (10 µM), 1 µL Reverse Primer (10 µM), 50 ng Template DNA, and nuclease-free water added to constant volume. The PCR reaction parameters were as follows: 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. The PCR products were detected via 1% agarose gel electrophoresis. The PCR mixture was recovered using an E.Z.N.A. ® Gel Extraction Kit (Omega, USA) Gel recovery Kit, and the target DNA fragment was eluted with TE buffer. The DNA concentration was measured using a Nano-drop ND-1000 spectrophotometer, and PCR products were sequenced by the Illumina Nova 6000 platform at Majorbio (PE = 250) platform of Guangdong Magigene Biotechnology Co., Ltd. (Guangzhou, China). The raw sequences were uploaded in the National Center for Biotechnology Information (NCBI) with the accession number PRJNA797190.
Sequence data processing and statistical analysis
The raw sequence data were processed according to the standard operating procedure (SOP) of Mothur software version 1.45.3 [32]. Under this SOP, after merging the reads R1 and R2, a sequence will be truncated if it contains: (i) ambiguous (N) bases; (ii) homopolymers longer than eight nucleotides; (iii) an average quality score lower than 25; (iv) chimeras (detected using Chimera uchim in Mothur); and (v) fewer than 200 nucleotides. The commands used to denoise and clean the sequences included fastp v.0.14.1 to remove the adapter and barcode sequences, trim.seqs to check the quality of sequencing errors, and pcr.seqs and chimera.uchime to check PCR errors and chimeras, respectively. Then, the sequences were pre-clustered with 6 bp differences by the Mothur pre-clustering method, and were clustered with 97% similarity to form operational taxonomic units (OTUs) [33, 34]. OTU with frequencies of less than 10 in all samples were deleted. The sequences were assigned to taxonomic groups with an 80% bootstrap confidence using the RDP Naïve Bayesian rRNA Classifier tool version 2.0. [35]. Sequences assigned to the plant chloroplast and non-bacteria domain were filtered out. Functional Annotation of Prokaryotic Taxa (FAPROTAX) was used to predict the biogeochemical cycle of the environmental samples, especially the cycle of carbon, nitrogen, phosphorus, with the default output function table, and we compared the dataset obtained by classification and the automatic function classifier FAPROTAX (script version 1.1 and database version 1.0) to determine the function group [36].
A total of 5,323,959 sequences were obtained after denoising and quality control, and the number of sequences of the samples ranged from 46,409 to 79,003, with an average of 59,155 ± 5011 (mean ± standard deviation). The minimum sample size of all samples (46,409) was used for random sampling and diversity indices calculation, including. Species richness estimation (Sobs, observed species), community diversity (Invsimpson), and community evenness (Simpson evenness) [37]. The centered log-ratio (CLR) transformation was used to convert value on raw abundance of functions data. One-way (ANOVA) and multivariate analysis of variance (MANOVA) were used to compare the differences in diversity and function structure of wood-inhabiting bacterial communities in different biochar treatments. The visualization and detection of differences in community structure and function were based on Canonical analysis of principal coordinates (CAP) based on Bray–Curtis dissimilarity at OTU level was used as ordination method and permutational analysis of variance (PERMANOVA) with Bray–Curtis dissimilarity after 9999 permutations. Venn diagrams (http://bioinformatics.psb.ugent.be/webtools/Venn/) were constructed using normalized data, which can intuitively show shared and unique OTUs among multiple samples. The relationship between the community structure or functional structure and the environmental factors was detected by distance-based linear model (DistLM) (selection procedure was exhaustive search using all variable combinations, and selection criteria was Akaike's Information Criterion (ACI), and the outcome p values in the supplementary table based on forcing inclusion of all specified variables). All the analyses were carried out by using PRIMER 7 [38]and SPSS.22.
Results
Wood decomposition rate in different biochar treatments
The wood decomposition rate did not differ between the biochar treatments and the control in both the first and the second year (Fig. 1). The wood decomposition rates decreased with the increase in biochar pyrolysis temperature, and increased with biochar application amount (Fig. 1). In the five treatments, with the extension of wood degradation time, the degradation rate of wood increased significantly (P < 0.05) (Additional file 1: Table S2).
Wood loss after one and two years of decomposition in different biochar treatments. The number before and after "https://link.springer.com/" represents the pyrolysis temperature of the biochar (°C) and application amount of the biochar (kg m−2), Lowercase letters were used to indicate the significant difference (P < 0.05) among the five treatments within the same year after biochar application
Wood-inhabiting bacteria community diversity in different biochar treatments
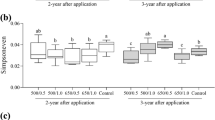
After one year of decomposition, the wood-inhabiting bacterial diversity in the 650 °C biochar treatment was significantly higher than the other treatments, respectively (P < 0.05) (Fig. 2a). This increased diversity relative to the other treatments persisted in the second year for the 650 °C /1.0 kg m−2 treatment. In the second year, differences also emerged in the 500 °C treatments, with increased diversity found with the 1.0 kg m−2 biochar treatment (P < 0.05) (Fig. 2a). The bacterial species richness did not differ between treatments during the decomposition period (Fig. 2b). After one-year decomposition, the bacterial evenness in the 500 °C biochar treatments was significantly lower than that in the control (P < 0.05) (Fig. 2c), but no differences were found among the treatments in the second year. Multivariate analysis of variance showed that the pyrolysis temperature, wood decomposition time, and the interaction between the pyrolysis temperature and the amount of biochar significantly affected the bacterial diversity (Additional file 1: Tables S2, S3).
The wood-inhabiting bacterial community a diversity, b richness, and c evenness after one and two years of decomposition in different biochar treatments. The number before and after "https://link.springer.com/" represents the pyrolysis temperature of biochar (°C) and application amount of biochar (kg m−2), Lowercase letters were used to indicate the significant difference (P < 0.05) among the five treatments within the same year after biochar application. Sobs: observed species, invsimpson: invers simpson
Wood-inhabiting bacterial community structure at the taxonomic level in different biochar treatments
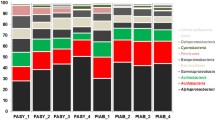
All sequences were divided into bacterial domains, and assigned to 16 141 OTUs, of which 98.9% of sequences were classified into 29 bacterial phyla and 445 bacterial genera. Proteobacteria (46.1%) was the most abundant phylum, followed by Actinobacteria (15.8%), Acidobacteria (15.7%), Planctomycetes (7.4%), Bacteroidetes (4.6%), and Verrucomicrobia (3.8%) (Fig. 3a).
The relative abundance of wood-inhabiting bacteria at the phylum level (a) and the top 10 most abundant genera (b) after one and two years of decomposition in different biochar treatments. The number before and after "https://link.springer.com/" represents the pyrolysis temperature of biochar (°C) and application amount of biochar (kg m−2)
After one year of decomposition, under the treatment with 1.0 kg m−2 biochar, the treatments with biochar produced at 500 °C had significantly lower abundance of Acidobacteria and Planctomycetes(7.17% and 4.07%), than the control (13.78 and 9.30%) (P < 0.05). And the abundance of Acidobacteria on the areas with the biochar applying amount of 1.0 kg m−2, more decreased in the treatment with biochar produced at 500 °C (7.17%) compared to that at 650 °C (12.31%). With the biochar produced at 650 °C, the treatment with 1.0 kg m−2 (16.81%) biochar had a higher abundance of Actinobacteria than that of the 0.5 kg m−2 (10.43%) (P < 0.05). For the 0.5 kg m−2 biochar application, the treatment with biochar produced at 650 °C (17.00%) increased the abundance of Acidobacteria compared to that produced at 500 °C (10.43%) (P < 0.05), while the abundance of Actinobacteria (10.43%) was lower than that at 500 °C (16.61%) (P < 0.05). For the biochar produced at 650 °C, the 1.0 kg m−2 treatment (16.81%) increased the abundance of Actinobacteria more than the 0.5 kg m−2 treatment (10.43%) (P < 0.05), and the abundance of Actinobacteria showed the same trend in the second year. The abundance of Proteobacteria in the treatments with biochar produced at 500 °C (0.5 kg m−2 and 1.0 kg m−2, 39.97% and 44.49%) decreased significantly in the second year compared to the first year (0.5 kg m−2 and 1.0 kg m−2, 50.01% and 54.13%) (P < 0.05). The detailed data on the relative abundance of the phyla (top 10) wood-inhabiting bacterial communities were listed in Additional file 1: Table S4.
The abundant genera included Edaphobacter (7.2%), Singulisphaera (5.6%), Burkholderia (3.4%), Mycobacterium (3.1%), Acidobacterium (2.7%), Sphingomonas (1.7%), Pseudomonas (1.6%), Acidothermus (1.2%), Mucilaginibacter (1.2%), and Rhizobium (1.1%) (Fig. 3b). After one year of decomposition, in the treatments with biochar produced at 650 °C, the abundance of Edaphobacter were significantly higher in application with the amount of 0.5 kg m−2 (9.41%) than that of the control (6.14%) and other biochar treatments (500 °C/0.5 kg m−2, 500 °C/1.0 kg m−2and 650 °C/1.0 kg m−2, 4.41%, 2.40% and 4.68%) (P < 0.05). With the application amount of 1.0 kg m−2, the abundance of Sphingomonas and Corynebacterinea were significantly increased in the treatment with biochar produced at 650 °C (4.71% and 5.04%) compared to that produced at 500 °C (2.32% and 1.83%) (P < 0.05), while that the abundance of Burkholderia and Sphingomonas were significantly lower than that the treatment at 500 °C (6.56% and 6.33%) (P < 0.05).
After two years of decomposition, with the biochar produced at 650 °C, the abundance of Acidothermus was significantly higher in the 0.5 kg m−2 treatment (3.81%) than in the 1.0 kg m−2 treatment (3.16%) (P < 0.05), while that the abundance of Sphingomonas were significantly lower than that than in the 1.0 kg m−2 treatment (0.97%) (P < 0.05). With 1.0 kg m−2 application, the abundance of Burkholderia was significantly increased with biochar produced at 650 °C (5.26%) compared to 500 °C (1.99%) (P < 0.05). The detailed data on the relative abundance of the genera (top 15) wood-inhabiting bacterial communities were listed in Additional file 1: Table S4.
Wood-inhabiting bacterial community structure at the OTU level in different biochar treatments
The unique and shared OTUs between different treatments are shown in Fig. 4, demonstrating that 22.2% and 20.7% of the OTUs were shared among the biochar treatments in the first and second year, respectively. The number of shared and unique OTUs in different biochar treatments showed a similar pattern in the first and second year. The number of unique OTUs in biochar treatments increased with the increase of decomposition time, except with the 500 °C/0.5 kg m−2 treatment (Fig. 4).
Venn diagram showing the wood-inhabiting bacteria unique and shared OTUs between different biochar treatments after one (a) and two years (b) of decomposition. The number before and after "https://link.springer.com/" represents the pyrolysis temperature of biochar (°C) and application amount of biochar (kg m−2)
CAP analysis based on the OTU data showed that the biochar treatments and the control formed distinct wood-inhabiting bacteria communities (P < 0.05) in both the first and second year (Fig. 5a). However, the treatments with biochar produced at different temperatures and with different biochar amounts did not form separate bacterial communities. Moreover, the wood-inhabiting bacterial community also differed between the first and second year. The difference in bacterial community structures was confirmed by PERMANOVA (Table 1). The DistLM using measured soil parameters as explanatory variables showed that the soil pH, soil organic matter (SOM), and soil total nitrogen (STN) were positively correlated with the bacterial communities in the second year (P < 0.05), in which the soil pH was correlated with the biochar treatment, while the SOM and STN were correlated with the control. The soil water content (SW) was positively correlated with the bacterial communities of biochar treatments in the first year (P < 0.05) (Fig. 5a, Additional file 1: Table S5).
Distance Based Linear Model (DistLM) showing the a wood-inhabiting bacterial community structure and b functional structure using environmental factors as explanatory variables after one and two years of decomposition in different biochar treatments. The significance of the green circled areas different bacterial community structures and functional groups were formed in different years of wood degradation; the significance of the yellow circled areas different bacterial community structures and functional groups were formed between biochar treatment and blank control in the same year of wood degradation
Wood-inhabiting bacterial community structure of the predicted function in different biochar treatments
3558 OTUs (25.7% of the total OTUs) were assigned to 39 functional groups using FAPROTAX. Chemoheterotrophy (57.9%) was the most abundant functional group, followed by ureolysis (13.2%), intracellular parasites (7.9%), methylotrophy (3.7%), hydrocarbon degradation (3.5%), methanotrophy (3.5%), phototrophy (2.4%), and cyanobacteria (2.4%) (Fig. 6).
The most abundant functional groups of wood-inhabiting bacteria after one and two years of decomposition in different biochar treatments. The number before and after "https://link.springer.com/" represents the pyrolysis temperature of biochar (°C) and application amount of biochar (kg m−2), respectively
After one year of decomposition, with 0.5 kg m−2 biochar application amount, the abundance of chemoheterotrophy increased significantly in the treatment with biochar produced at 650 °C (65.88%) than in that produced at 500 °C (55.55%) (Fig. 7a). The treatment with biochar produced at 650 °C, the abundance of ureolysis, methylotrophy and intracellular parasites (13.20%, 4.41% and 10.11%) in the biochar application amount of 1.0 kg m−2 were significantly higher than that of 0.5 kg m−2 (8.53%, 4.22% and 6.09%) (Fig. 7b–d), while the abundance of chemoheterotrophy (52.21%) was significantly lower than that of 0.5 kg m−2 treatment (65.88%) (P < 0.05) (Fig. 7a).
Centered Log-Ratio (CLR) transformation converted value of functional groups of wood-inhabiting bacteria (a) chemoheterotrophy (b) ureolysis (c) methylotrophy (d) intracellular parasites in different treatments, Lowercase letters were used to indicate the significant difference (P < 0.05) among the five treatments within the same year after wood decomposition. The number before and after "/" represents the pyrolysis temperature of biochar (°C) and applying amount of biochar (kg m−2), respectively
After two years of decomposition, no significant effect were observed among biochar treatments with different pyrolysis temperatures and the amount of biochar different on the abundance of functional groups (Fig. 7a–d). The detailed data on the relative abundance of the function (top 10) of wood-inhabiting bacterial communities were listed in Additional file 1: Table S6. CAP analysis based on the functional data showed that the biochar treatments and the control formed distinct bacterial functional structures in the first year (P < 0.05), but not in the second year (Fig. 5b). Moreover, the bacterial functional structure also differed between the first and second year. Subsequent PERMANOVA confirmed the differences in community structures (Table 1). Similarly, the soil total nitrogen (STN) and soil organic matter (SOM) were positively correlated with bacterial functional structures in the second year (P < 0.05). The soil pH and soil water content (SW) were positively correlated with bacterial functional structures of the biochar treatments in the first year (P < 0.05) (Fig. 5b, Additional file 1: Table S5).
Discussion
In this study, the wood-inhabiting bacterial structure and function during the wood decomposition under different biochar treatments were studied. Treatments with biochar produced at 650 °C had higher bacterial diversity than did those with biochar produced at 500 °C. Biochar has the characteristics of high carbon content, high pH, and high porosity, which can change the physical and chemical properties of soil after application, such as the soil nutrients and soil pH [2, 39]. One of the most important elements determining the pH and surface area of biochar is the pyrolysis temperature, through which the soil CO2 emissions, including microbial and root respiration, are affected [2, 40,41,42]. The biochar produced at high temperatures had a higher pH [29], which might increase the pH value of the soil, benefiting most soil bacteria [40, 43, 44]. A previous study has shown that biochar mixed with soil can significantly increase the soil pH [44]. In our study, the biochar was spread on the top of the vegetation rather than mixed with the soil directly, to avoid soil disturbance. This might partly explain some of the observations in our study in that although the soil pH in all the biochar treatments increased, only one biochar treatment of 500 °C/1.0 kg m−2 showed a significantly higher soil pH compared to the control.
The treatments with biochar produced at different temperatures and different application amounts significantly affected the abundance of certain wood-inhabiting bacterial taxa during the decomposition process, e.g., Acidobacteria and Actinobacteria. Acidobacteria can spread widely in various environments (i.e., the ocean and activated sludge), demonstrating general adaptability and functional diversity [45]. In this study, one year after beginning decomposition, the abundance of Acidobacteria in the areas with a biochar application amount of 1.0 kg m−2 decreased more in the treatment with biochar produced at 500 °C (7.17%) compared to 650 °C (12.31%). A previous study showed that soil pH may become a limiting factor for soil microbial growth following biochar application [46], In this study, the soil pH was significantly higher in the 500 °C/1.0 kg m−2 treatment than the other treatments (Additional file 1: Table S1), moreover, the relative abundance of Acidobacteria was negatively correlated with soil pH in the 500/1.0 treatment (Additional file 1: Table S7), which might affect the abundance of Acidobacteria. In addition, Acidobacteria from soil produce a higher amount of lignin decomposing enzyme, which may contribute to the degradation of polysaccharides in wood [47]. Most members of the Actinobacteria family can be associated with nutrient cycling, and can degrade cellulose and chitin as the main resource for the soil nutrient supply [48]. The abundance of Actinobacteria was significantly higher in the 1.0 kg m−2 treatment (16.81%) than in the 0.5 kg m−2 treatment (10.43%) with biochar produced at 650 °C. Actinobacteria can produce a series of extracellular enzymes to effectively decompose complex aromatic substances [49]. In this study, at pyrolysis temperature of 650 °C, under the high application amount of biochar, the relative abundance of Actinobacteria positively was correlated with soil pH, which promoted the growth of Actinobacteria (Additional file 1: Table S7). Li et al. also showed that biochar applied at both 2 kg m−2 and 6 kg m−2 to the soil in a China fir plantation significantly increased the proportion of Actinobacteria 90 days after application [50].
The treatments with 1.0 kg m−2 biochar, and the treatments with biochar produced at 650 °C (4.71%) significantly increased the relative abundance of Sphingomonas after one year of decomposition, compared to that produced at 500 °C (2.32%) after one year decomposition. Members of the genus Sphingomonas have an aerobic heterotrophic soil-based lifestyle, with an additional ability to degrade extraordinarily recalcitrant carbon sources and to produce related exopolysaccharides [51]. In the early decomposition process of wood, Sphingomonas is a common genus that uses simple carbon compounds [52]. The biochar produced by pyrolysis at a high temperature is rich in ash content [53], in this study, in the treatment with 650 °C/1.08 kg m−2, the abundance of Sphingomonas was positively correlated with soil organic matter, which promoted the growth of Sphingomonas (Additional file 1: Table S7). Burkholderia has the ability to degrade recalcitrant xenobiotics [54]. With the 1.0 kg m−2 biochar treatment, the abundance of Burkholderia significantly increased in the treatment with biochar produced at 650 °C (5.26%) compared to that produced at 500 °C (1.99%) after two years of decomposition. Burkholderia can be involved in the decay of forest litter in peat forest soil [12]. With increasing pyrolysis temperature of the biochar, the content of aromatic carbon in the biochar increases, as does the content of refractory carbon [18]. In this study, there is a close positive correlation between the abundance of Burkholderia and ureolysis with 650 °C/1.0 kg m−2 (Additional file 1: Table S7). Moreover, Fraver et al. showed that the most of the wood structures in the late stage of decomposition have obviously disintegrated and more recalcitrant substances remain [55], which might contribute to the increase in the abundance of certain specialized microbes.
The biochar treatments formed distinct wood-inhabiting bacterial communities during the decomposition process. Bacteria are more sensitive to unstable substrates and unstable carbon in biochar can directly affect the growth of bacteria [56]. The wood-inhabiting bacteria structure also differed during the decomposition period. This is consistent with other studies, showing that the microbial community structure changes as wood decomposition proceeds [57]. At the early stage of wood degradation, bacteria can use some easily available substances, such as polysaccharides. The wood components change with increasing degradation time, driving a shift in the microbial community [17, 58]. In addition, fresh biochar increases the activity of soil microorganisms and stimulate the decomposition of wood due to the labile carbon components on its surface [8, 44].
Similar to the bacterial community structure, the bacterial functional structure differed between the biochar treatments and the control in the first year of decomposition, but not in the second year. The change of bacterial community structure can reflect the change of bacterial community function to a certain extent, but in terms of the microbial functional redundancy, this is not always the case. Moreover, in our case, the functional analysis was based only on a small portion of the OTUs data, which may not reflect the true situation. Biochar can change the composition of understory vegetation, especially when mixed with soil [6]. The treatment with biochar produced at 650 °C, and 1.0 kg m−2 biochar application amount the abundance of methylotrophy (4.41%) were significantly higher than that in the 0.5 kg m−2 treatment (4.22%) The functional of methylotrophy can directly reflect the ability of bacteria degrade carbon-related organic matters. [59]. Biochar application can affect microbes directly by providing a large amount of carbon, or indirectly [2, 60]. At higher pyrolysis temperature and higher application rate, the abundance of microorganisms related to carbon cycle, such as methyl nutrition groups, was increased, thus improving the nutrient cycle efficiency and accelerating the process of carbon cycle [60].
Both biochar application and wood decomposition are long and complicated processes, and in this study, we only monitored the situation for two years after biochar application. In addition, fungi also contribute significantly to litter degradation, and the interaction between microbes during wood degradation is important. Therefore, longer term monitoring is needed to understand the full picture of wood-inhabiting microbes during wood decomposition after biochar application in boreal forests.
Conclusion
The interaction between the biochar pyrolysis temperature and the amount of biochar applied significantly affected the wood-inhabiting bacterial diversity (P < 0.05). With the extension of the decomposition time, the wood-inhabiting bacterial diversity and species richness decreased. Biochar application shifted the wood-inhabiting bacterial community during the wood decomposition process, despite the different biochar pyrolysis temperatures and application amounts. Similarly, biochar application shifted the wood-inhabiting bacterial function in the first year of degradation. Proteobacteria, Actinobacteria, Acidobacteria, Edaphobacter, Singulisphaera, and Burkholderia were the most abundant wood-inhabiting bacterial taxa after biochar application, and the abundance was affected by the biochar pyrolysis temperature and application amount during the decomposition process. Long-term monitoring is needed to better understand the effects of biochar application on the wood-inhabiting microbial community in boreal forests.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available in the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) with the following accession number: PRJNA797190. The material is original and the manuscript has not been submitted for publication elsewhere while under consideration for publication in Environmental Microbiome, and has not been previously published.
References
Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. Adv Agron. 2010;105:47–82.
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota—a review. Soil Biol Biochem. 2011;43(9):1812–36.
Zhou G, Zhou X, Zhang T, Du Z, He Y, Wang X, et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For Ecol Manag. 2017;405:339–49.
Zwieten LV, Kimber S, Morris S, Chan KY, Downie A, Rust J, et al. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil. 2010;327(1):235–46.
Zhao P, Palviainen M, Köster K, Berninger F, Bruckman VJ, Pumpanen J. Effects of biochar on fluxes and turnover of carbon in boreal forest soils. Soil Sci Soc Am J. 2019;83(1):126–36.
Grau-Andrés R, **ree MRA, Öquist MG, Wardle DA, Nilsson MC, Gundale MJ. Biochar increases tree biomass in a managed boreal forest, but does not alterN2O, CH4, and CO2 emissions. GCB Bioenergy. 2021;13(8):1329–42.
Sponseller RA, Gundale MJ, Futter M, Ring E, Nordin A, Nasholm T, et al. Nitrogen dynamics in managed boreal forests: recent advances and future research directions. Ambio. 2016;45(Suppl 2):175–87.
Palviainen M, Aaltonen H, Laurén A, Köster K, Berninger F, Ojala A, et al. Biochar amendment increases tree growth in nutrient-poor, young Scots pine stands in Finland. For Ecol Manag. 2020;474:118362.
Robertson SJ, Rutherford PM, López-Gutiérrez JC, Massicotte HB. Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can J Soil Sci. 2012;92(2):329–40.
Simpson AJ, Simpson MJ, Smith E, Kelleher BP. Microbially derived inputs to soil organic matter: are current estimates too low? Environ Sci Technol. 2007;41(23):8070–6.
Dilly O, Bloem J, Vos A, Munch JC. Bacterial diversity in agricultural soils during litter decomposition. Appl Environ Microbiol. 2004;70(1):468–74.
Sun H, Terhonen E, Koskinen K, Paulin L, Kasanen R, Asiegbu FO. Bacterial diversity and community structure along different peat soils in boreal forest. Appl Soil Ecol. 2014;74:37–45.
Boer WD, Wal AJBMSSS. Chapter 8 Interactions between saprotrophic basidiomycetes and bacteria. 2008;28(08):143–53.
Clausen CA. Bacterial associations with decaying wood: a review. Int Biodeterior Biodegrad. 1996;37(1–2):101–7.
Rossell SE, Abbot EGM, Levy JF. Bacteria and wood: a review of the literature relating to the presence, action and interaction of bacteria in wood. Inst Wood Sci J. 1973;6(2):28–35.
Sun H, Terhonen E, Kasanen R, Asiegbu FO. Diversity and community structure of primary wood-inhabiting bacteria in boreal forest. Geomicrobiol J. 2014;31(4):315–24.
Purahong W, Wubet T, Lentendu G, Schloter M, Pecyna MJ, Kapturska D, et al. Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol Ecol. 2016;25(16):4059–74.
Li Y, Hu S, Chen J, Müller K, Li Y, Fu W, et al. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soils Sediments. 2017;18(2):546–63.
Palansooriya KN, Wong JTF, Hashimoto Y, Huang L, Rinklebe J, Chang SX, et al. Response of microbial communities to biochar-amended soils: a critical review. Biochar. 2019;1(1):3–22.
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H. Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ. 2015;206:46–59.
Novak JM, Lima I, **ng B, Gaskin JW, Schomberg H. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci. 2009;3:195–206.
Genevieve L, Noyce N, Basiliko R, et al. Soil microbial responses over 2 years following biochar addition to a north temperate forest. Biol Fertil Soils. 2015;51:649–59.
Wardle DA, Nilsson MC, Zackrisson O. Fire-derived charcoal causes loss of forest humus. Science. 2008;320(5876):629.
Minamino Y, Fujitake N, Suzuki T, Yoshitake S, Koizumi H, Tomotsune M. Effect of biochar addition on leaf-litter decomposition at soil surface during three years in a warm-temperate secondary deciduous forest, Japan. Sci Rep. 2019;9(1):16961.
Abiven S, Andreoli R. Charcoal does not change the decomposition rate of mixed litters in a mineral cambisol: a controlled conditions study. Biol Fertil Soils. 2011;47(1):111–4.
Palviainen M, Pumpanen J, Berninger F, Ritala K, Duan B, Heinonsalo J, et al. Nitrogen balance along a northern boreal forest fire chronosequence. PLoS ONE. 2017;12(3):e0174720.
Cajander AK. Forest types and their significance. Acta For Fenn. 1949;56(5):1–71.
Pirinen P, Simola H, Aalto J, Kaukoranta JP, Ruuhela R. Tilastoja Suomen ilmastosta 1981–2010 (Climatological statistics of Finland 1981–2010). FinnMeteorol Inst Rep. 2012;1:91–6.
Palviainen M, Berninger F, Bruckman VJ, Köster K, de Assumpção CRM, Aaltonen H, et al. Effects of biochar on carbon and nitrogen fluxes in boreal forest soil. Plant Soil. 2018;425(1–2):71–85.
Bruckman VJ, Varol EA, Uzun BB, Liu J. Biochar: a regional supply Chain approach in view of climate change mitigation. England: Cambridge University Press; 2016.
Nan X, Tan G, Wang H, Gai X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol. 2016;74:1–8.
Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE. 2011;6(12): e27310.
Needleman SB. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–53.
Nilsson SG, Hedin J, Niklasson M. Biodiversity and its assessment in boreal and nemoral forests. Scand J For Res. 2010;16(sup003):10–26.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7.
Parfrey L, Wegener L, Stilianos D, et al. Decoupling function and taxonomy in the global ocean microbiome. Science. 2016;353(6305):1272–7.
Ma Y, Qu ZL, Liu B, Tan JJ, Asiegbu FO, Sun H. Bacterial community structure of Pinus Thunbergii naturally infected by the Nematode Bursaphelenchus Xylophilus. Microorganisms. 2020;8(2):1–16.
Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: guide to software and statistical methods. England: PRIMER-E, Plymouth; 2008.
Liu C, Wang H, Tang X, Guan Z, Reid BJ, Rajapaksha AU, et al. Biochar increased water holding capacity but accelerated organic carbon leaching from a slo** farmland soil in China. Environ Sci Pollut Res Int. 2016;23(2):995–1006.
Lehmann J. Bio-energy in the black. Front Ecol Environ. 2007;5:381–7.
Hawthorne I, Johnson MS, Jassal RS, Black TA, Grant NJ, Smukler SM. Application of biochar and nitrogen influences fluxes of CO2, CH4 and N2O in a forest soil. J Environ Manag. 2017;192:203–14.
Smith JL, Collins HP, Bailey VL. The effect of young biochar on soil respiration. Soil Biol Biochem. 2010;42(12):2345–7.
Kamble PN, Gaikwad VB, Kuchekar SR, Bååth E. Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India). Eur J Soil Biol. 2014;65:87–95.
Zhu X, Zhu T, Pumpanen J, Palviainen M, Zhou X, Kulmala L, et al. Short-term effects of biochar on soil CO2 efflux in boreal Scots pine forests. Ann For Sci. 2020;77(2):59–74.
Ellen R, Graber Y, Meller H, et al. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil. 2010;337:481–96.
Kolb SE, Fermanich KJ, Dornbush ME. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J. 2009;73(4):1173–81.
Lladó S, Žifčáková L, Větrovsky T, Eichlerová I, Baldrian P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol Fertil Soils. 2016;52(2):251–60.
Huang K-C, Shen Y-Y, Xu G-P, Huang Y-Q, Zhang D-N, Sun Y-J, et al. Effects of reclamation on soil nutrients and microbial activities in the huixian karst wetland in Guilin. Environ Sci. 2018;39(4):1813–23.
Mccarthy AJ, Williams ST. Actinomycetes as agents of biodegradation in the environment—a review. Gene. 1992;115(1–2):189–92.
Li Y, Zhou C, Qiu Y, Tigabu M, Ma X. Effects of biochar and litter on carbon and nitrogen mineralization and soil microbial community structure in a China fir plantation. J For Res. 2018;30(5):1913–23.
White DC, Sutton SD, Ringelberg DB. The genus Sphingomonas: physiology and ecology. Curr Opin Biotechnol. 1996;7(3):301–6.
Vojtěch T, Petra Z, Tomá V, et al. Bacteria associated with decomposing dead wood in a natural temperate forest. FEMS Microbiol Ecol. 2017;93(12):1–13.
Zhu X, Chen B, Zhu L, **ng B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut. 2017;227:98–115.
Coenye T, Vandamme P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol. 2003;5(9):719–29.
Fraver S, Milo AM, Bradford JB, D’Amato AW, Kenefic L, Palik BJ, et al. Woody debris volume depletion through decay: implications for biomass and carbon accounting. Ecosystems. 2013;16(7):1262–72.
Ameloot N, Graber ER, Verheijen FGA, De Neve S. Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci. 2013;64(4):379–90.
Purahong W, Kapturska D, Pecyna MJ, Jariyavidyanont K, Kaunzner J, Juncheed K, et al. Effects of forest management practices in temperate beech forests on bacterial and fungal communities involved in leaf litter degradation. Microb Ecol. 2015;69(4):905–13.
Zhao Y, Wu F, Yang W, Tan B, He W. Variations in bacterial communities during foliar litter decomposition in the winter and growing seasons in an alpine forest of the eastern Tibetan Plateau. Can J Microbiol. 2016;62(1):35–48.
Yu Y, Liu L, Wang J, Zhang Y, **ao C. Effects of warming on the bacterial community and its function in a temperate steppe. Sci Total Environ. 2021;792:148409.
Liang S, Deng J, Jiang Y, Wu S, Zhou Y, Zhu W. Functional distribution of bacterial community under different land use patterns based on FAPROTAX function prediction. Pol J Environ Stud. 2020;29(2):1245–61.
Acknowledgements
This study was funded by The Foundation for Research of Natural Resources in Finland (2016085), the Academy of Finland (286685, 294600, 277623), and the FCoE of atmospheric sciences (Center of Excellence (1118615). CSC (IT Center for Science Ltd., Finland, https://www.csc.fi) is kindly acknowledged for the provision of generous computational resources. We would also like to thank the staff of the Hyytiälä Forestry Field Station for supporting us with the field work.
Funding
The National Natural Science Foundation of China (31870474). The Foundation for Research of Natural Resources in Finland (2016085). The Academy of Finland (286685, 294600, 307222, 277623). The FCoE of atmospheric sciences (Center of Excellence (1118615)).
Author information
Authors and Affiliations
Contributions
JP, JH, FB and HS: conceptualization. XlL, ZLQ, YG, and HS: methodology and writing—original draft preparation. HS, XZ, XlL and ZLQ: software, formal analysis, and data curation. JP, KK, FB, JH, MP and HS: writing—review and editing. JP, KK, MP and HS: funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
The soil physical chemical properties under different biochar treatments. Table S2 Multivariate analysis of variance showing the difference in wood loss rate, bacterial community diversity, richness and evenness under different biochar treatments by using the pyrolysis temperatures, applying amounts and wood degradation time since application as variates. Table S3 Difference of application amount of biochar at the same pyrolysis temperature on diversity of bacteria in the same degradation year. Table S4 The relative abundance (%) of the phyla (top 10) and the genera (top 15) of wood-inhabiting bacteria in different biochar treatments. Table S5 DistLM showing the correlation between the soil properties and the wood-inhabiting bacterial community and functional structures. Table S6 The relative abundance (%) of the function (top 10) of wood-inhabiting bacteria in different biochar treatments. Table S7 Correlation between relative abundance (%) of Acidobacteria, Actinobacteria, Singulisphaera, Burkholderia and wood degradation rate, soil physical and chemical properties and some functions during wood degradation under different biochar treatments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qu, Zl., Li, Xl., Ge, Y. et al. The impact of biochar on wood-inhabiting bacterial community and its function in a boreal pine forest. Environmental Microbiome 17, 45 (2022). https://doi.org/10.1186/s40793-022-00439-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-022-00439-9