Abstract
We report the high production of stilbenes, including resveratrol and viniferin, in grapevine (Vitis labruscana L.) cell cultures through elicitation with methyl jasmonate (MeJA) and stevioside (STE). Methyl-β-cyclodextrin (MeβCD) is widely used as a solubilizer for resveratrol production. For the first time, we used STE as a solubilizer for stilbene production in plant cell cultures. MeJA was most effective elicitor in activating VvSTS expression and stimulating stilbene biosynthesis in grapevine cell cultures. The maximum concentration of δ-viniferin (892.2 mg/L) production with a small amount of trans-resveratrol (12.2 mg/L) was observed in the culture medium by co-treatment of cells with MeJA and STE, whereas the highest level of trans-resveratrol (371.9 mg/L) with a slight amount of δ-viniferin (11.5 mg/L) was accumulated in the culture medium of cells treated with MeJA and MeβCD. However, neither trans-resveratrol nor δ-viniferin were significantly elevated within the cells by the applications. Notably, predominant production of δ-viniferin and trans-resveratrol was observed in shake and static flask culture medium, respectively, by co-treatment of MeJA and STE. Furthermore, stilbene compounds of resveratrol, ε-viniferin, and δ-viniferin were mainly produced in a 3-L bioreactor culture following elicitation of cells with MeJA and STE. These results provide new strategies for conditional, high-level production of resveratrol and viniferin in cell cultures by utilizing the solubilizing properties of STE or MeβCD.

Similar content being viewed by others
Introduction
Plants synthesize a wide range of secondary metabolites in response to various environmental stresses (Langcake and Pryce 1977; Zamboni et al. 2006). Stilbenes, particularly resveratrol and viniferin, have attracted extensive attention and interest due to their health benefits. Grapevines produce stilbenes derived from the phenylpropanoid pathway. Plant stilbenes are phytoalexins that accumulate in a small number of plant species in response to biotic and abiotic stresses and are mainly derivatives of the monomeric unit trans-resveratrol (trans-3,5,4′-trihydroxystilbene) (Donnez et al. 2011; Jeandet et al. 2002, 2020; Sotheeswaran and Pasupathy 1993). Resveratrol is a naturally occurring stilbene produced in more than 70 plant species, including grapevines, peanuts, and Japanese knotweed (Aggarwal et al. 2004; Bhat and Pezzuto 2002; Jang et al. 1997; Jeong et al. 2016). The biosynthesis of resveratrol is controlled by stilbene synthase (STS). UV-irradiation induces accumulation of stilbenes by inducing STS gene expression in grape berries (Pan et al. 2009; Pezet et al. 2003). Methyl jasmonate (MeJA) also induces accumulation of resveratrol and ε-viniferin, a resveratrol dehydrodimer in grapevines (Vezzulli et al. 2007). In addition, pathogen infection stimulates biosynthesis of δ- and ε-viniferin in grapevine leaves (Bavaresco et al. 1997; Pezet et al. 2003). In most plants producing stilbenes, STS genes exist as a family of closely related genes. Genome-wide analysis of the STS gene family based on the grapevine PN40024 genome revealed the identification of 48 putative STS genes, designated VvSTS1 to VvSTS48, with at least 33 full-length coding genes (Jaillon et al. 2007; Vannozzi et al. 2012). Expression of VvSTS genes is differentially regulated by biotic and abiotic stresses, pathogen infection, mechanical wounding or hormones (Almagro et al. 2014; Chialva et al. 2018; Lijavetzky et al. 2008; Vannozzi et al. 2012).
Resveratrol is the skeleton for producing various derivatives. Piceid and pinostilbene or pterostilbene are produced through glycosylation and methylation of resveratrol by UDP-glycosyltransferases (UGT) and resveratrol O-methyl transferases (ROMT), respectively, while oxidative dimerization of two resveratrol units by (4-hydroxystilbene) peroxidases leads to production of δ- or ε-viniferin (Jeandet et al. 2002; Jeong et al. 2014, 2015; Xue et al. 2014). Resveratrol has been implicated in a large number of beneficial effects on human health (Bradamante et al. 2004; Goswami and Das 2009; Valenzano et al. 2006). Due to its therapeutic value, it is in demand for nutraceutical, cosmetic, and pharmaceutical applications.
Resveratrol and its derivatives are currently produced by extraction from plant materials, chemical synthesis, and bio-production (Donnez et al. 2009; Jeandet et al. 2016). Two major biotechnological approaches are currently widely applied in bio-production: (i) the use of genetically engineered microorganisms or plants (Donnez et al. 2009, 2011; Jeandet et al. 2018; Wang and Yu 2012; Wu et al. 2013) and (ii) the use of in vitro plant cell cultures under controlled conditions (Chastang et al. 2018; Donnez et al. 2009, 2011; Jeandet et al. 2016), especially grapevine cell cultures. The bio-production of resveratrol and its derivatives in grapevine cell cultures has been reported as a promising biotechnological alternative to their plant extraction and chemical synthesis. Plant cell cultures have been used extensively for bio-production of valuable secondary metabolites under controlled conditions independent of climatic changes or soil conditions (Giri and Zaheer 2016; Hussain et al. 2012; Jeandet et al. 2016; Jeong et al. 2018; Mulabagal and Tsay 2004; Murthy et al. 2014). To date, elicitation with MeJA combined with dimethyl-β-cyclodextrins (MeβCD) has proven the most efficient approach for high production of resveratrol and its derivatives in grapevine cell cultures (Bru et al. 2006; Lijavetzky et al. 2008; Martinez-Esteso et al. 2009; Martínez-Márquez et al. 2016) (Donnez et al. 2009; Lambert et al. 2019). Furthermore, it has been shown that stevioside (STE), a diterpene glycoside comprising an aglycone (steviol) and three molecules of glucose with mono- and disaccharide carbohydrate residues at the C13 and C19 positions, possesses solubilizing properties (Liu 2011; Uchiyama et al. 2012; Wan et al. 2013).
The aim of this study was to investigate the effects of solubilizing agents such as MeβCD and STE in combination with MeJA as the most effective elicitor on the bio-production of resveratrol and viniferin in grapevine cell cultures. Herein, we show that the combined applications of MeJA and MeβCD or STE offer improved and preferential production of resveratrol and viniferin in cell culture media. This is the first study showing that STE can be used as a solubilizer in combination with MeJA to trigger increased production of viniferin in grapevine cell culture medium. Moreover, we suggest that the use of MeβCD and STE with solubilizing properties in plant cell cultures makes culture systems more reliable and productive for induced biosynthesis and secretion of secondary metabolites.
Materials and methods
Plant material
Grapevine (Vitis labruscana L. cv. Campbell Early) was obtained from a commercial vineyard in Korea for this study. Under aseptic conditions, the anther explants were surface-sterilized with 70% (v/v) ethanol for 30 s, subsequently soaked in a 20% (v/v) solution of commercial sodium hypochlorite (NaClO) containing 4% (w/v) active ingredient for 15–20 min, and rinsed three times with sterile distilled water before being dried on sterile filter paper.
Callus induction and cell suspension cultures
The calli were induced from the anther explants of grapevines on MS1D (Murashige and Skoog basal salt mixture supplemented with 1.0 mg/L 2,4-D and 3% (w/v) sucrose) medium containing 4 g/L Gelrite (Duchefa Biochemie B.V., Haarlem, The Netherlands) in darkness at 24 °C. The homogenous calli selected from callus cultures were maintained by transferring the relatively friable portion of a callus onto MS1D solid medium every 4 weeks. Finally, the best calli were deposited at the Korean Collection for Type Cultures (KCTC; http://bioproduct.kribb.re.kr) as bio-product BP1347372. Grapevine cell suspension cultures were initiated and established by inoculating small pieces of homogeneous and friable calli in MS1D liquid medium as described by Jeong et al. (2018). The cell cultures were agitated at 90 rpm on a rotary shaker and maintained by sub-culturing every 4 weeks in MS1D liquid medium in darkness at 24 °C. The cultured cells were harvested via filtration through a nylon mesh filter (100 µm), freeze-dried, and weighed to determine grams of dry weight (DW).
Cell suspension growth curve
A cell growth curve was used to determine the most suitable period for harvesting. Cell growth was measured based on dry weight of cultures. For this experiment, approximately 2.5 g fresh weight (FW) of the established cells (i.e., 7 days old), were transferred into 25-mL of fresh medium in 125 mL Erlenmeyer flasks. To determine cell density at each time point, the harvested cells were collected and dried at 60 °C. The dry weight of the cultured cells was determined after drying the harvested cells. The cell suspension cultures were monitored at 2-day intervals for 16 days. Measurements were repeated three times.
Elicitation of grapevine cell suspension cultures
Cell suspension cultures were sub-cultured into 100-mL MS1D liquid medium in 500-mL Erlenmeyer flasks and placed on a rotary shaker (90 rpm) at 24 °C under dark conditions for 7 days as described by Jeong et al. (2018). For RT-PCR and qRT-PCR analyses, pre-cultured cells (6 mL aliquots) were transferred into each well of a 6-well tissue plate. Twelve hours later, the cells were treated for 48 h with various elicitors, such as methyl jasmonate (MeJA), salicylic acid (SA), abscisic acid (ABA), ethephon (ET), flagellin 22 peptide (Flg22), methyl viologen (MV), and chitosan (Chi). For stilbene production in 500-mL flask cultures, cells (approximately 10 g) pre-cultured in 100-mL MS1D liquid medium for 7 days were treated with 100 µM MeJA and/or 50 mM stevioside (Daepyung, Ltd., Seongnam, Korea) or 50 mM methyl-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA) for 5 days. In bioreactor cultures, cells (approximately 10 g/100 mL, 3 flasks) pre-cultured for 14 days were transferred into a 3-L bioreactor containing 1.2-L MS1D medium. After cultivation for 5 days, the cells were treated for 5 days with 100 µM MeJA and/or 50 mM STE. The elicited samples (cells and medium) were harvested by filtration through a nylon mesh filter (100 µm). The elicitation experiments were carried out with three replicates. The specific growth rate (μ) was calculated according to Eq. (1) where X0 and X are the initial and final biomasses (g/L), respectively, and \({{\Delta }}t\) is the culture time interval (h) (Homova et al. 2010):
Volume specific productivity (Qp) is defined as the stilbenes produced per cell volume during a period of time (mgL−1h−1). It was calculated according to Eq. (2) where P, V and t represent the product concentration, cell volume and time, respectively (Maurer et al. 2006):
Monitoring of cell viability
Cell viability was monitored by staining cell suspensions with 0.1% Evans blue (Sigma-Aldrich) as previously described by Jeong et al. (2018). For cell viability assay, cells (approximately 10 g) pre-cultured in 100-mL MS1D liquid medium for 7 days were treated with 100 μΜ MeJA and/or 50 mM STE or 50 mM MeβCD for 5 days. Cell viability was calculated by examining the ratio of viable (unstained) and non-viable (stained blue) cells under light microscope.
RNA extraction and quantitative RT-PCR (qRT-PCR) analysis
Total RNA isolation, cDNA synthesis, and RT-PCR were performed as previously described (An et al. 2015; Jeong et al. 2018). The cDNA was diluted 50-fold, and 1 μL of the diluted cDNA was used in a 20 μL PCR reaction. qRT-PCR was performed using the TransStart Tip Green qPCR Supermix. Assays were performed using a CFX96 real-time PCR detection system (Bio-Rad, CFX96) with thermal parameters of 95 °C for 15 min, followed by 45 cycles at 95 °C for 20 s and 60 °C for 40 s. Transcript levels were calculated after normalization against the beta-actin gene (VvActin7, XM_002282480.4) as an internal control. Relative changes in gene expression levels were determined by the 2−ΔΔCt method (Livak and Schmittgen 2001). Data were analyzed and presented as average values ± standard deviation (n = 3). The primers used in this study are shown in Additional file 1: Table S1.
Resveratrol and viniferin extraction and HPLC analysis
For analysis of resveratrol and viniferin contents in the callus cells of grapevine cell cultures, freeze-dried calli (approximately 1 g) were extracted with 5 mL of 80% methanol. After extraction, samples were concentrated by evaporation and dissolved in 0.5 mL 80% methanol. For analysis of stilbenes produced in cell culture medium, 1 mL of the culture medium was extracted with an equal volume of ethyl acetate, and samples were concentrated by evaporation and dissolved in 1 mL 80% methanol. The extract samples were purified using a 0.2-µm hydrophilic PTFE membrane filter (Advantec MFS, Inc., Dublin, CA, USA) before HPLC analysis. The concentration of trans-resveratrol, ε-viniferin, and δ-viniferin produced by grapevine cell cultures was calculated by comparison with the known concentrations (mg/L) of trans-resveratrol, ε-viniferin (Sigma-Aldrich), and δ-viniferin as standards. Calibration curves (R2 = 0.999) were prepared by using five different concentrations of trans-resveratrol and viniferin from 0.005 to 0.5 mg/mL.
δ-Viniferin concentration was determined using the compound isolated from the grapevine cell cultures for use as a standard. For isolation and identification of resveratrol and viniferins, the grapevine cell cultures were extracted with ethyl acetate at room temperature for a week and the combined extracts were concentrated in vacuo to give a dark residue (15 g). The dark residue was fractionated over a reverse-phase silica gel column (ODS-A, 12 nm, S-150 μm, YMC) with a gradient of H2O–acetonitrile (100:1 → 0:100, v/v) to yield seven fractions (Fr. A-Fr. G) based on TLC profile. Fraction C (1.3 g) and Fraction D (2.2 g) were separated and purified by column chromatography on Sephadex LH-20 (mobile phase: MeOH), reverse-phase silica gel, and preparative HPLC to yield resveratrol and viniferins. All the chromatographic procedure for the isolation was performed as described previously (Vitrac et al. 2005). The structures of the isolated compounds were confirmed by comparing their physical and spectroscopic data (1H NMR and 13C NMR; Additional file 1: Figs. S4 and S5) with the known structures of viniferins (Pezet et al. 2003; Sako et al. 2004; Vitrac et al. 2005). Concentration is shown at the milligrams per liter scale (mg/L). δ-Viniferin and ε-viniferin were identified by NMR analysis (Additional file 1: Figs. S4 and S5).
HPLC and LC–MS analyses were performed using an Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA) as previously described by Jeong et al. (2018). Each stilbene compound was identified by comparison with commercial standards. The quantity of stilbene compounds in each sample was determined from the standard curve.
Results
Establishment and optimization of grapevine cell cultures
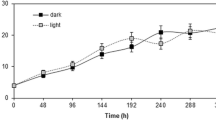
The grapevine cell suspension cultures were established using calli derived from anthers and maintained by sub-culturing homogeneous cells in MS1D liquid medium several times at 2-week intervals. As shown in Fig. 1, grapevine cell growth exhibited a similar growth pattern as that observed in other plant cell suspension cultures (Muatafa et al. 2001). An initial lag phase was observed during the first 1–4 days, followed by an exponential rise in cell growth until day 12, with maximum cell density on day 12 (approximately 17.3 g DW). During the stationary phase (12–16 days), cell density declined after day 12 along with a gradual decrease in cell viability. This observation indicated that 4–8 days of culture in the early exponential phase was likely the best time to harvest cells for subsequent experiments.
Expression analysis of stilbene synthase genes following elicitor application
Resveratrol biosynthesis is controlled by stilbene synthase (STS), which controls the entry point into the stilbene biosynthetic pathway (Fig. 2a). Recent study reported that several VvSTS genes in grapevine cell suspension cultures are differentially expressed in response to different elicitors such as MeJA and ethylene (Chialva et al. 2018). Thus, to obtain the best conditions for resveratrol production in grapevine cell suspension cultures, the expression patterns of the grapevine VvSTS genes (VvSTS6, VvSTS7, VvSTS36, and VvSTS42) were analyzed using specific primers by RT-PCR and qRT-PCR using flask culture samples treated with MeJA, SA, ABA, and ET as elicitors (Fig. 2b; Additional file 1: Fig. S1). As shown in Fig. 2b, all of these VvSTS genes were most highly induced 3 h after treatment with MeJA, indicating that MeJA was most effective elicitor in activating the expression of those VvSTS genes in grapevine cell cultures. VvSTS6, VvSTS7, and VvSTS36 genes reached their peaks (more than 40 folds) 3 h after MeJA elicitation, followed by a gradual decrease until 48 h. VvSTS42 exhibited the highest and most significant induction (more than 100-fold), reaching a peak 12 h after MeJA elicitation, maintained this level until 48 h. VvSTS7 and VvSTS36 also responded to SA, ABA, and ET application. However, VvSTSs did not respond to the application of Flg22, MV, or chitosan (Additional file 1: Fig. S1). Higher levels of VvSTS expression were observed in cell suspension cultures elicited with 100 μM MeJA than in those elicited with 300 μM MeJA (Additional file 1: Fig. S1). These results indicate that VvSTS expression levels in MeJA-treated cells were significantly higher than those in SA-, ABA- or ET-treated cells. This is in agreement with the work of Chialva et al. (2018) who observed that the VvSTS genes were significantly induced by MeJA treatment in grapevine suspension cultures. In this study, we thus used 100 μM MeJA as an elicitor for the induction of resveratrol production in the grapevine cell cultures.
a Proposed stilbene biosynthetic pathway in grapevines and b effects of various elicitors on stilbene synthase genes (VvSTS6, VvSTS7, VvSTS36, and VvSTS42) expression in grapevine cell cultures. The cells were pre-cultured for 7 days and harvested at different time points after elicitation for qRT-PCR analysis. The elicitors used are as follows: MeJA, methyl jasmonate; SA, salicylic acid; ABA, abscisic acid; ET, ethephon. VvActin7 (XM_002282480.4) was used as a quantitative control. The sequential actions of PAL, C4H, 4CL, STS, and UGT or PRX result in the conversion of phenylalanine to stilbenes. PAL, phenylalanine ammonia-lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumarate-CoA-ligase; STS, stilbene synthase; UGT, UDP-glucosyltransferase; ROMT, resveratrol O-methyltransferase; PRX, peroxidase
Enhanced production of resveratrol and viniferin in grapevine cell cultures
We further examined whether up-regulation of VvSTS by MeJA application induces enhanced production of stilbenes, such as resveratrol and piceid. Resveratrol and piceid production has been reported in grapevine cell cultures upon stimulation of cells by several elicitors, including MeJA (Almagro et al. 2014; Belchí-Navarro et al. 2012; Belhadj et al. 2008; Donnez et al. 2011; Lijavetzky et al. 2008; Martínez-Márquez et al. 2016). Thus, endogenous accumulation within the cells and release into the culture medium of resveratrol and its derivatives were measured in grapevine cell cultures treated with 100 μM MeJA. Figure 3 shows that the accumulation of trans-piceid, a glycosylated form of trans-resveratrol, considerably increased within the cells when MeJA was added to grapevine cell cultures. However, no significant increase in trans-resveratrol or trans-piceid contents was detected after treatment with ABA or SA. The maximum trans-piceid production of 54.647 µg/g DW was observed 48 h after treatment with 100 μM MeJA. We noticed that trans-piceid production was detected in the cells but not in the culture medium (data not shown).
Effects of various elicitors on accumulation of stilbene compounds in grapevine cell cultures. The cells were pre-cultured for 7 days and harvested 2 days after elicitation with the indicated elicitors. Samples were extracted with methanol and subjected to HPLC analysis. HPLC analysis was performed using a C18 reverse-phase column at 300 nm. Chromatograms t-RES and t-PIC represent the authentic standards trans-resveratrol and t-piceid with retention times of 17.200 and 13.568 min, respectively. Con, untreated control. Chromatogram MeJA indicates the t-piceid production in the elicited cells 2 days after elicitation with 100 µM MeJA
Previous studies (Lijavetzky et al. 2008; Liu 2011; Wan et al. 2013) reported that cyclodextrin and stevioside effectively enhance the solubility of water-insoluble resveratrol. Thus, we investigated whether trans-resveratrol or trans-piceid production can be improved by the use of solubilizing agents, such as MeβCD and STE, in the cell cultures (Fig. 4).
Extracellular production of trans-resveratrol and δ-viniferin in the grapevine cell culture medium. a, b Extracellular production of trans-resveratrol and δ-viniferin in grapevine cell culture medium after treatment with different solubilizers. Cells were pre-cultured for 7 days and harvested 5 days after co-application of 100 µM MeJA and 50 mM MeβCD or 50 mM STE as a solubilizer. c Time-course of δ-viniferin production in grapevine cell culture medium after co-treatment with MeJA and STE. d, e Water solubility enhancement of trans-resveratrol and δ-viniferin in STE solution. The solubility of trans-resveratrol (blue) and δ-viniferin (red) in aqueous solution with increasing STE concentrations (%, w/v) was determined. (Insert) Images of trans-resveratrol (RES) and δ-viniferin (VIN) dispersion with 0.5% STE concentration (w/v). The culture medium of the elicited cells was extracted with ethyl acetate and analyzed by HPLC
As shown in Fig. 4 and Additional file 1: Fig. S3, trans-resveratrol production was considerably increased by addition of both MeJA and MeβCD in the culture medium, but not in the cells, reaching 371.9 mg/L 5 days after elicitation in shake flask cultures. However, trans-piceid was not detected in both the cells and culture medium by co-treatment with MeJA and MeβCD, even though it was accumulated within MeJA-treated cells as shown in Fig. 3. Interestingly, co-treatment with MeJA and STE greatly induced high-level production of δ-viniferin as a major compound along with a small amount of trans-resveratrol in the culture medium, but not in the cells. As shown in Fig. 4c, the maximum production of δ-viniferin (892.2 mg/L) in the extracellular medium was observed on day 5 following elicitation of the cells with MeJA and STE in shake flask cultures. Unlike application of MeJA and/or MeβCD, we observed that the cell cultures gradually turned brown following co-treatment with MeJA and STE, indicating the accumulation of viniferin (Fig. 4a). These results suggest that MeβCD and STE preferentially allow release of trans-resveratrol and δ-viniferin, respectively, into the culture medium.
Since δ-viniferin production in culture medium was greatly enhanced by STE application, we examined whether the solubilizing effect of STE on water-insoluble resveratrol and viniferin is concentration-dependent. As shown in Fig. 4d, e, STE effectively induced solubilization of hydrophobic trans-resveratrol and δ-viniferin in water solutions. The solubilities of trans-resveratrol and δ-viniferin in water without STE were 92.0 µg/mL and 419.7 µg/mL, respectively. As STE concentration increased to approximately 2.5% (w/v), higher solubility was observed for δ-viniferin than for trans-resveratrol. The solubility of δ-viniferin was enhanced at even low concentrations (0.1–0.6%) of STE (Fig. 4e). In the presence of 1% STE (w/v), trans-resveratrol and δ-viniferin were solubilized in water to about 309.0 µg/mL and 897.8 µg/mL, respectively. Most trans-resveratrol and δ-viniferin (more than 90%) were solubilized in approximately 3% and 1% STE (w/v) solutions, respectively. These results suggest that STE effectively enhances the water solubility of water-insoluble trans-resveratrol and δ-viniferin.
Conditional production of trans-resveratrol and δ-viniferin in grapevine cell cultures
As shown in Fig. 4, the combined use of MeJA and STE predominantly produced δ-viniferin along with a trace amount of resveratrol in shake flask culture medium. Interestingly, we found that δ-viniferin production was greatly reduced while trans-resveratrol accumulation was highly enhanced under stationary flask culture (Fig. 5; Additional file 1: Fig. S3). Furthermore, we noticed that a mixture of stilbenes, including trans-resveratrol (149 mg/L), ε-viniferin (1213 mg/L), and δ-viniferin (2583 mg/L) were produced in a 3-L bioreactor culture 5 days following elicitation of cells with MeJA and STE (Fig. 5). The calculated specific growth rate (μ) was 0.005 h−1 for a 10% by volume inoculum in 3% sucrose. In addition, the volume specific productivity (Qp) was 0.51 mgL−1h−1, 4.21 mgL−1h−1 and 8.97 mgL−1h−1 for the resveratrol, ε-viniferin and δ-viniferin, respectively. These results suggest that stilbene production (resveratrol or viniferin) is influenced by cultivation conditions in grapevine cell cultures. In other words, it is possible to obtain specific compounds by controlling cultivation conditions (with or without shaking) for the preferential production of resveratrol or viniferin in flask cultures. We also examined the electrochemical properties of the isolated trans-resveratrol and viniferins by cyclic voltammetry analysis (Additional file 1: Fig. S6). Cyclic voltammograms displayed two oxidation peaks which were irreversible. They also exhibited that the oxidation potential of trans-resveratrol was higher than those of ε-viniferin and δ-viniferin.
Conditional production of trans-resveratrol, ε-viniferin, and δ-viniferin in grapevine cell culture medium under different culture conditions. a Phenotypes of grapevine cell cultures 5 days after co-treatment with 100 µM MeJA and 50 mM STE in flask (shaking or stationary) and bioreactor cultures. b HPLC chromatograms (300 nm) of ethyl acetate extract from the culture medium of the elicited cell cultures showing production of resveratrol and viniferin in flask and bioreactor cultures. For flask and bioreactor cultures of grapevine cells, cells were pre-cultured for 7 or 14 days in 500-mL Erlenmeyer flask and in a 3-L bioreactor, respectively, and harvested 5 days after co-treatment with 100 µM MeJA and 50 mM STE
As shown in Figs. 4a and 5a, the cultured cells became a darker brown color 5 days post-elicitation with MeJA and STE, unlike untreated control cells or cells elicited with MeJA and MeβCD, indicating the accumulation of oxidation products. Cell growth was also observed to be somewhat reduced by the application of MeJA and STE. Application of MeJA and STE alone exhibited about 4% and 7% reduction in grapevine cell growth (estimated by DW biomass), respectively. Co-treatment with MeJA and STE showed about 8% decrease in the cell growth. This application probably caused some cell death (Additional file 1: Fig. S2), but the brown color may have been the result of accumulation of viniferin, oxidized dimers of resveratrol, produced during cell cultures. In general, resveratrol and viniferin appear white and brown, respectively. As shown in Additional file 1: Fig. S2B, the application of MeJA alone or MeJA and STE or MeβCD exhibited approximately 91% to 95% viability of grapevine cells 5 days after elicitation. STE treatment alone displayed slightly lower viability (~ 83%) compared to the untreated control (~ 96%). However, there were no significant differences among numbers of blue-stained (non-viable) cells observed after treatment with MeJA alone or MeJA and STE or MeβCD compared to that in untreated control cells. These results indicate that the concentrations of MeJA and STE or MeβCD used in this study were not cytotoxic.
Discussion
In recent years, resveratrol has attracted considerable attention as a star polyphenol compound due to its abundant health benefits. Resveratrol oligomers also have numerous impressive bioactivities, but few studies have been performed due to their trace amounts in natural resources (Shen et al. 2009). Thus, there have been many efforts to enhance production of resveratrol and its derivatives in plants and microorganisms through metabolic engineering (Beekwilder et al. 2006; Delaunois et al. 2009; Donnez et al. 2009; Jeong et al. 2014, 2015, 2016; Nivelle et al. 2017; Shin et al. 2011; Yu et al. 2006). Currently, plant cell suspension cultures are a promising approach for resveratrol production under controlled aseptic conditions. This biotechnological production is commonly coupled with cell culture-based biotransformation using an exogenous supply of biosynthetic precursors or various elicitors.
Elicitation of plant cells has been shown to be the most efficient way to induce and boost the biosynthesis of useful secondary metabolites (Namdeo 2007; Zhao et al. 2005). To date, there have been many reports that MeJA application can effectively promote resveratrol production in grapevine cell suspension cultures (Almagro et al. 2014; Belchí-Navarro et al. 2012; Belhadj et al. 2008; Donnez et al. 2009; Lambert et al. 2019; Lijavetzky et al. 2008; Martínez-Márquez et al. 2016; Tassoni et al. 2005). Furthermore, Tassoni et al. (2005) reported that MeJA effectively stimulated endogenous accumulation of resveratrol and its release into the culture medium. In this study, we established an efficient method for producing trans-resveratrol and δ-viniferin in culture medium (i.e., extracellular compartment) of grapevine cell cultures using MeβCD and STE as solubilizers. Upon MeJA elicitation, MeβCD and STE were highly effective in promoting accumulation of trans-resveratrol and δ-viniferin in the culture medium, respectively (Fig. 4). However, there was no accumulation or release of trans-resveratrol or δ-viniferin into the culture medium in the absence of MeβCD or STE. Our data represent the first report on the extracellular production of δ-viniferin in culture medium of MeJA-treated cells using STE as a solubilizer. Additionally, the use of MeβCD allowed the cells to release trans-resveratrol into the culture medium in response to MeJA application (Fig. 4). MeβCD treatment alone induced a small amount of trans-resveratrol (10 mg/L) and δ-viniferin (0.3 mg/L) (Additional file 1: Fig. S3). Likely, MeβCD is thus able to encapsulate trans-resveratrol, but it is also considered as an elicitor. These results suggest that the combined treatment of MeJA and MeβCD had synergistic effect on the production of trans-resveratrol and viniferin. Unlike MeβCD, however, STE alone was hardly able to induce the VvSTS expression and resveratrol biosynthesis as an elicitor (data not shown). Previous studies have demonstrated that cyclodextrins promote the accumulation and release of resveratrol in plant cell cultures (Bru et al. 2006; Donnez et al. 2009; Lambert et al. 2019; Lijavetzky et al. 2008; Morales et al. 1998). We are also the first to report that STE facilitates production and secretion of δ-viniferin into the culture medium. Until now, there has been no experimental evidence showing how STE promotes secretion of trans-resveratrol and δ-viniferin to the extracellular medium in grapevine cells or any other plant cell systems. We speculate that STE enabled secretion of trans-resveratrol into the extracellular medium (i.e., apoplastic space) via uncharacterized membrane transporters in response to MeJA elicitation. After being transported out of cells, the resveratrol monomer might undergo oxidative dimerization to form resveratrol dimers of viniferin due to oxidizing conditions. Several types of membrane transporters, including ATP-binding cassette (ABC) transporters, are known to be involved in the secondary metabolite transport process (Grotewold 2004; Yazaki 2005). However, further investigation will be needed to gain insight into the transport of trans-resveratrol and δ-viniferin out of the cells in grapevine cell cultures. Unlike cyclodextrins, the STE–resveratrol complex likely favors itself with access to extracellular oxidizing enzymes (e.g., peroxidases and polyphenol oxidases) present in the extracellular compartment (i.e., apoplastic space), thereby leading to the formation of viniferin in the culture medium. Indeed, Calderon et al. (1994) previously reported that a cell wall-bound peroxidase catalyzes dimerization of resveratrol to form viniferin in grapevine cell suspension cultures. In addition, Wan et al. (2013) demonstrated that STE effectively improves the water solubility of resveratrol by incorporation of a water-soluble STE-resveratrol complex. In the present study (Fig. 4; Additional file 1: Fig. S3), we also showed that STE has a similar solubilizing effect on water-insoluble viniferin as well as resveratrol. It has been shown that STE has characteristic properties of micelle-like nanostructures that allow it to self-assemble into micelles in aqueous solutions (Liu 2011; Uchiyama et al. 2012; Wan et al. 2013; Zhang et al. 2011). Likely, we found that rebaudioside A, a steviol glycoside, also increased the water solubility of trans-resveratrol and δ-viniferin. However, it exhibited relatively lower water solubility of trans-resveratrol and δ-viniferin than STE (data not shown). These characteristics of the STE structure likely include the combination of hydrophobic diterpene and hydrophilic sugar side chains. It was hypothesized that there is an interaction between the hydrophobic core of STE and resveratrol in STE self-assembled micelles that enhances the solubility of resveratrol. In our study, for the first time, we applied the biosurfactant STE as a solubilizer to enhance the extracellular production of resveratrol in a plant cell culture system. Thus, it would be very interesting to investigate whether STE similarly enhances the solubility of other polyphenolic compounds, such as curcumin and paclitaxel, with poor water solubility in plant cell cultures. Furthermore, compared to MeβCD (98% purity: ~ $400/kg), STE (98% purity: ~ $70/kg) is an inexpensive substance and commercially available with high purity, so it can be readily used as a solubilizer for extracellular production of stilbenes in plant cell cultures.
We also observed δ-viniferin production in flask cultures supplied with oxygen by shaking, whereas trans-resveratrol was produced in stationary cultures without agitation after the cells were treated with MeJA and STE (Fig. 5). As described above, these findings suggest that STE encapsulates the resveratrol synthesized in the elicited cells within STE micelles, which then secrete resveratrol complexes into the extracellular compartment, where resveratrol might be converted to viniferin by oxidative dimerization. Interestingly, we also noticed that δ-viniferin was mainly produced in shake flask cultures upon elicitation with MeJA and STE, while ε-viniferin was produced in a 3-L bioreactor culture in addition to trans-resveratrol and δ-viniferin. It seems that the grapevine cell cultures in the bioreactor might synthesize the ε- and δ-viniferin in addition to trans-resveratrol because the bioreactor culture undergoes more shear stress resulting from agitation and aeration compared to the flask culture. Donnez et al. (2011) demonstrated that grapevine cell cultures (41B cells) are very sensitive to agitation speed and aeration. They also observed production of resveratrol (209 mg/L) with small amounts of ε- and δ-viniferin in the medium of MeJA-elicited cell cultures in a 2-L bioreactor. More recently, Lambert et al. (2019) reported that co-treatment of MeJA and MeβCD in Vitis labrusca cell suspension cultures induced high level of the extracellular production of trans-resveratrol (6140 mg/L), δ-viniferin (540 mg/L), and ε-viniferin (500 mg/L) in a 5-L bioreactor. In the present study (Fig. 5), the high production level of trans-resveratrol (149 mg/L), ε-viniferin (1213 mg/L), and δ-viniferin (2583 mg/L) in a 3-L bioreactor was detected in the culture medium upon elicitation with MeJA and STE, suggesting that they were secreted from cells in high quantities. As suggested above, however, the mechanism of viniferin biosynthesis by certain peroxidases remains to be elucidated.
By further optimization of plant cell culture conditions and metabolic pathway engineering strategies, production of resveratrol and viniferin could be further enhanced. For example, it would be quite interesting to examine whether the addition of resveratrol precursors, such as phenylalanine or p-coumaric acid, to the culture medium would increase the productivity of grapevine cell cultures. In the future, scaling of grapevine cell cultures to the industrial level will need to be achieved and optimized to produce large quantities of trans-resveratrol as well as ε- and δ-viniferin using MeJA and STE.
Conclusions
We found that MeJA was most effective elicitor in activating the expression of VvSTSs genes and stimulating stilbene biosynthesis in grapevine cell cultures. For the first time, we used STE as a solubilizer for the bio-production of secondary metabolites in plant cell cultures. The use of MeJA in combination with MeβCD or STE represents an effective elicitation strategy for enhancing the production of trans-resveratrol and δ-viniferin, respectively, in grapevine cell cultures, as well as their high production and secretion outside cells in the culture medium. In particular, the solubilizer STE could be applied for enhanced production and secretion of other water-insoluble metabolites/substances in plant cell cultures.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary information files.
Abbreviations
- MeJA:
-
Methyl jasmonate
- STE:
-
Stevioside
- MeβCD:
-
Methyl-β-cyclodextrin
- UGT:
-
UDP-glycosyltransferase
- ROMT:
-
Resveratrol O-methyl transferase
- DW:
-
Dry weight
- FW:
-
Fresh weight
- SA:
-
Salicylic acid
- ABA:
-
Abscisic acid
- ET:
-
Ethephon
- Flg22:
-
Flagellin 22 peptide
- MV:
-
Methyl viologen
- Chi:
-
Chitosan
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- HPLC:
-
High-performance liquid chromatography
- STS:
-
Stilbene synthase
References
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24:2783–2840
Almagro L, Carbonell-Bejerano P, Belchí-Navarro S, Bru R, Martínez-Zapater JM, Lijavetzky D, Pedreño MA (2014) Dissecting the transcriptional response to elicitors in Vitis vinifera cells. PLoS ONE 9:e109777
An CH, Lee KW, Lee SH, Jeong YJ, Woo SG, Chun H, Park Y-I, Kwak S-S, Kim CY (2015) Heterologous expression of IbMYB1a by different promoters exhibits different patterns of anthocyanin accumulation in tobacco. Plant Physiol Biochem 89:1–10
Bavaresco L, Petegolli D, Cantù E, Fregoni M, Chiusa G, Trevisan M (1997) Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 36:77–83
Beekwilder J, Wolswinkel R, Jonker H, Hall R, de Vos CHR, Bovy A (2006) Production of resveratrol in recombinant microorganisms. Appl Environ Microbiol 72:5670–5672
Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA (2012) Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep 31:81–89
Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Mérillon J-M (2008) Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46:493–499
Bhat KPL, Pezzuto JM (2002) Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci 957:210–229
Bradamante S, Barenghi L, Villa A (2004) Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev 22:169–188
Bru R, Sellés S, Casado-Vela J, Belchí-Navarro S, Pedreño MA (2006) Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J Agric Food Chem 54:65–71
Calderon AA, Zapata JM, Ros Barcelo A (1994) Peroxidase-mediated formation of resveratrol oxidation products during the hypersensitive-like reaction of grapevine cells to an elicitor from Trichoderma viride. Physiol Mol Plant Pathol 44:289–299
Chastang T, Pozzobon V, Taidi B, Courot E, Clément C, Pareau D (2018) Resveratrol production by grapevine cells in fed-batch bioreactor: experiments and modelling. Biochem Eng J 131:9–16
Chialva C, Muñoz C, Miccono M, Eichler E, Calderón L, Prieto H, Lijavetzky D (2018) Differential expression patterns within the grapevine stilbene synthase gene family revealed through their regulatory regions. Plant Mol Biol Rep 36:225–238
Delaunois B, Cordelier S, Conreux A, Clément C, Jeandet P (2009) Molecular engineering of resveratrol in plants. Plant Biotechnol J 7:2–12
Donnez D, Jeandet P, Clément C, Courot E (2009) Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol 27:706–713
Donnez D, Kim K-H, Antoine S, Conreux A, De Luca V, Jeandet P, Clément C, Courot E (2011) Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process Biochem 46:1056–1062
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tiss Org Cult 126:1–18
Goswami SK, Das DK (2009) Resveratrol and chemoprevention. Cancer Lett 284:1–6
Grotewold E (2004) The challenges of moving chemicals within and out of cells: insights into the transport of plant natural products. Planta 219:906–909
Homova V, Weber J, Schulze J, Alipieva K, Bley T, Georgiev M (2010) Devil’s claw hairy root culture in flasks and in a 3-L bioreactor: bioactive metabolite accumulation and flow cytometry. Z Naturforsch C 65:472–478
Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad I (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4:10–20
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Jeandet P, Douillet-Breuil A-C, Bessis R, Debord S, Sbaghi M, Adrian M (2002) Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 50:2731–2741
Jeandet P, Clément C, Tisserant L-P, Crouzet J, Courot É (2016) Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C R Chim 19:1062–1070
Jeandet P, Sobarzo-Sánchez E, Clément C, Nabavi SF, Habtemariam S, Nabavi SM, Cordelier S (2018) Engineering stilbene metabolic pathways in microbial cells. Biotechnol Adv 36:2264–2283
Jeandet P, Sobarzo-Sánchez E, Silva AS, Clément C, Nabavi SF, Battino M, Rasekhian M, Belwal T, Habtemariam S, Koffas M, Nabavi SM (2020) Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol Adv 39:107461
Jeong YJ, An CH, Woo SG, Jeong HJ, Kim Y-M, Park S-J, Yoon BD, Kim CY (2014) Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzyme Microb Technol 54:8–14
Jeong YJ, Woo SG, An CH, Jeong HJ, Hong Y-S, Kim Y-M, Ryu YB, Rho M-C, Lee WS, Kim CY (2015) Metabolic engineering for resveratrol derivative biosynthesis in Escherichia coli. Mol Cells 38:318–326
Jeong YJ, An CH, Woo SG, Park JH, Lee K-W, Lee S-H, Rim Y, Jeong HJ, Ryu YB, Kim CY (2016) Enhanced production of resveratrol derivatives in tobacco plants by improving the metabolic flux of intermediates in the phenylpropanoid pathway. Plant Mol Biol 92:117–129
Jeong YJ, An CH, Park S-C, Pyun JW, Lee J, Kim SW, Kim H-S, Kim H, Jeong JC, Kim CY (2018) Methyl jasmonate increases isoflavone production in soybean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J Agric Food Chem 66:4099–4105
Lambert C, Lemaire J, Auger H, Guilleret A, Reynaud R, Clément C, Courot E, Taidi B (2019) Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 L bioreactor. Plants 8:576
Langcake P, Pryce RJ (1977) A new class of phytoalexins from grapevines. Experientia 33:151–152
Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA (2008) Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132
Liu Z (2011) Diterpene glycosides as natural solubilizers. US Patent Application PCT/US2011, 0033525
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Martinez-Esteso MJ, Sellés-Marchart S, Vera-Urbina JC, Pedreño MA, Bru-Martinez R (2009) Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv. Gamay) cell cultures in response to elicitors. J Proteomics 73:331–341
Martínez-Márquez A, Morante-Carriel JA, Ramírez-Estrada K, Cusidó RM, Palazon J, Bru-Martínez R (2016) Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures. Plant Biotechnol J 14:1813–1825
Maurer M, Kühleitner M, Gasser B, Mattanovich D (2006) Versatile modeling and optimization of fed batch processes for the production of secreted heterologous proteins with Pichia pastoris. Microb Cell Fact 5:37
Morales M, Bru R, García-Carmona F, Ros Barceló A, Pedreño MA (1998) Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tiss Org Cult 53:179–187
Mustafa NR, de Winter W, van Iren F, Verpoorte R (2011) Initiation, growth and cryopreservation of plant cell suspension cultures. Nat Protoc 6:715–742
Mulabagal V, Tsay H-S (2004) Plant cell cultures an alternative and efficient source for the production of biologically important secondary metabolites. J Appl Sci Eng Tech 2:29–48
Murthy HN, Lee E-J, Paek K-Y (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Org Cult 118:1–16
Namdeo A (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Nivelle L, Hubert J, Courot E, Jeandet P, Aziz A, Nuzillard J-M, Renault J-H, Clément C, Martiny L, Delmas D, Tarpin M (2017) Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 22:474
Pan Q-H, Wang L, Li J-M (2009) Amounts and subcellular localization of stilbene synthase in response of grape berries to UV irradiation. Plant Sci 176:360–366
Pezet R, Perret C, Jean-Denis JB, Tabacchi R, Gindro K, Viret O (2003) δ-Viniferin, a resveratrol dehydrodimer: one of the major stilbenes synthesized by stressed grapevine leaves. J Agric Food Chem 51:5488–5492
Sako M, Hosokawa H, Ito T, Iinuma M (2004) Regioselective oxidative coupling of 4-hydroxystilbenes: synthesis of resveratrol and ε-viniferin (E)-dehydrodimers. J Org Chem 69:2598–2600
Shen T, Wang X-N, Lou H-X (2009) Natural stilbenes: an overview. Nat Prod Rep 26:916–935
Shin S-Y, Han NS, Park Y-C, Kim M-D, Seo J-H (2011) Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate:coenzyme A ligase and stilbene synthase genes. Enzyme Microb Technol 48:48–53
Sotheeswaran S, Pasupathy V (1993) Distribution of resveratrol oligomers in plants. Phytochemistry 32:1083–1092
Tassoni A, Fornalè S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N (2005) Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol 166:895–905
Uchiyama H, Tozuka Y, Nishikawa M, Takeuchi H (2012) Nanocomposite formation between alpha-glucosyl stevia and surfactant improves the dissolution profile of poorly water-soluble drug. Int J Pharm 428:183–186
Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A (2006) Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 16:296–300
Vannozzi A, Dry IB, Fasoli M, Zenoni S, Lucchin M (2012) Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol 12:130
Vezzulli S, Civardi S, Ferrari F, Bavaresco L (2007) Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. Am J Enology Vitic 58:530–533
Vitrac X, Bornet A, Vanderlinde R, Valls J, Richard T, Delaunay JC, Mérillon JM, Teissédre PL (2005) Determination of stilbenes (ε-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, ε-viniferin) in Brazilian wines. J Agric Food Chem 53:5664–5669
Wan Z-L, Wang J-M, Wang L-Y, Yang X-Q, Yuan Y (2013) Enhanced physical and oxidative stabilities of soy protein-based emulsions by incorporation of a water-soluble stevioside–resveratrol complex. J Agric Food Chem 61:4433–4440
Wang Y, Yu O (2012) Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J Biotechnol 157:258–260
Wu J, Liu P, Fan Y, Bao H, Du G, Zhou J, Chen J (2013) Multivariate modular metabolic engineering of Escherichia coli to produce resveratrol from L-tyrosine. J Biotechnol 167:404–411
Xue Y-Q, Di J-M, Luo Y, Cheng K-J, Wei X, Shi Z (2014) Resveratrol oligomers for the prevention and treatment of cancers. Oxid Med Cell Longev 2014:9
Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8:301–307
Yu CKY, Lam CNW, Springob K, Schmidt J, Chu IK, Lo C (2006) Constitutive accumulation of cis -piceid in transgenic Arabidopsis overexpressing a sorghum stilbene synthase gene. Plant Cell Physiol 47:1017–1021
Zamboni A, Vrhovsek U, Kassemeyer H-H, Mattivi F, Velasco R (2006) Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 45:63–68
Zhang F, Koh GY, Jeansonne DP, Hollingsworth J, Russo PS, Vicente G, Stout RW, Liu Z (2011) A novel solubility-enhanced curcumin formulation showing stability and maintenance of anticancer activity. J Pharm Sci 100:2778–2789
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgements
We thank the members of the Korean Collection for Type Cultures (KCTC) at KRIBB for valuable supports of plant cell lines.
Funding
This work was supported by grants from the KRIBB Initiative Program and the Next-Generation BioGreen 21 Program (SSAC, Grant #: PJ01318604), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
CYK and JCJ conceived and supervised this study; CYK, JCJ, and YJJ designed the experiments; YJJ, SHP, THK, and SWK performed the grapevine cell cultures; YJJ and SCP performed the RT-PCR and qRT-PCR experiments; YJJ, SHP, and JL performed the Evans blue staining and water solubility experiments; YBR, SK, and SHP performed the HPLC, LC–MS, and NMR experiments. CYK, JCJ, and YJJ wrote the manuscript with assistance from the other authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors have read and agreed the ethics for publishing the manuscript.
Consent for publication
All authors approved the consent for publishing the manuscript to Bioresources and Bioprocessing.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, Y.J., Park, S.H., Park, SC. et al. Induced extracellular production of stilbenes in grapevine cell culture medium by elicitation with methyl jasmonate and stevioside. Bioresour. Bioprocess. 7, 38 (2020). https://doi.org/10.1186/s40643-020-00329-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-020-00329-3