Abstract
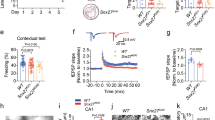
Mutations in the solute carrier family 6-member 8 (Slc6a8) gene, encoding the protein responsible for cellular creatine (Cr) uptake, cause Creatine Transporter Deficiency (CTD), an X-linked neurometabolic disorder presenting with intellectual disability, autistic-like features, and epilepsy. The pathological determinants of CTD are still poorly understood, hindering the development of therapies. In this study, we generated an extensive transcriptomic profile of CTD showing that Cr deficiency causes perturbations of gene expression in excitatory neurons, inhibitory cells, and oligodendrocytes which result in remodeling of circuit excitability and synaptic wiring. We also identified specific alterations of parvalbumin-expressing (PV+) interneurons, exhibiting a reduction in cellular and synaptic density, and a hypofunctional electrophysiological phenotype. Mice lacking Slc6a8 only in PV+ interneurons recapitulated numerous CTD features, including cognitive deterioration, impaired cortical processing and hyperexcitability of brain circuits, demonstrating that Cr deficit in PV+ interneurons is sufficient to determine the neurological phenotype of CTD. Moreover, a pharmacological treatment targeted to restore the efficiency of PV+ synapses significantly improved cortical activity in Slc6a8 knock-out animals. Altogether, these data demonstrate that Slc6a8 is critical for the normal function of PV+ interneurons and that impairment of these cells is central in the disease pathogenesis, suggesting a novel therapeutic venue for CTD.
Similar content being viewed by others
Introduction
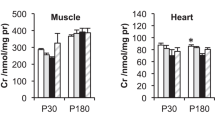
Creatine Transporter Deficiency (CTD) is an X-linked metabolic disorder causing cerebral creatine (Cr) deficit, intellectual disability, psycho-motor impairment, autistic-like behavior, and epilepsy. CTD etiology has been related to multiple mutations in the solute carrier family 6-member 8 (Slc6a8) gene encoding the protein responsible for cellular Cr uptake [1, 2].
Animal models either lacking the Slc6a8 gene or engineered to express an allele with a point mutation found in patients, largely reproduce the endophenotype of the human condition [3,4,5,6,7,8]. The availability of these transgenic lines of rodents allowed the scientific community to take initial steps towards dissecting the pathological determinants of CTD [8]. Loss-of-function of Slc6a8 does not result in overt alterations of brain structure and neuronal density, but rather induces a subtle reorganization of cerebral circuits and cellular metabolic processes [3, 5, 7,8,9,10,11,12,13,14,15]. However, a clear picture of the key cellular players involved in the development and progression of CTD is still missing. This represents a major issue, because a better knowledge of the causative mechanisms is crucial to identify novel druggable targets of translational value for a disease that is still untreatable [8].
Different cell types in the brain have distinctive metabolic profiles [16], resulting in a highly diversified energy demand in neuronal and glial populations [17]. Neurons consume 75–80% of the energy produced [18, 19] and might be particularly susceptible to the decreased ATP availability observed in CTD [9]. Intriguingly, the analysis of Slc6a8 RNA and protein levels revealed that its expression presents a significant heterogeneity across brain circuits [20,21,66,67,68,69], suggesting the possibility that an exaggerated synaptic pruning might undermine the solidity of brain circuits in CTD.
Conclusions
In summary, our study demonstrates that CTD pathogenesis is likely to have a complex multicellular profile with a potential network of cell-autonomous and non-autonomous effects, but the dysfunction of PV+ interneurons is a crucial mediator of the CTD neurological phenotype. Pharmacological manipulation of PV+ synapses can improve cortical processing in CrT−/y mice, indicating that therapeutic strategies selectively protecting PV+ interneurons should be explored to prevent and/or minimize their deterioration in CTD. Drugs targeting dysfunctional PV+ circuits are already available and have shown beneficial effects in other neuropsychiatric disorders such as schizophrenia, Fragile X syndrome and Rett syndrome [57, 70]. Our results can hopefully set the background for investigating the applicability of these compounds and, more in general, for drug repurposing for the treatment of CTD.
Availability of data and materials
The data supporting the conclusions of this article are present in the paper and the Supplementary Material. Raw data from RNA-seq experiments are available at the at the GEO repository GSE218797 for bulk sequencing and GSE216766 for snRNA sequencing. Requests for data and materials should be submitted to the last author.
Abbreviations
- AAV vector:
-
Adeno-associated viral vector
- ACC:
-
Anterior cingulate cortex
- BP:
-
Biological process
- CC:
-
Cellular component
- Cr:
-
Creatine
- CTD:
-
Creatine transporter deficiency
- DEGs:
-
Differentially expressed genes
- EEG:
-
Electroencephalography
- ER:
-
Endoplasmic reticulum
- fAHP:
-
Fast afterhyperpolarization
- GO:
-
Gene ontology
- IOS:
-
Intrinsic optical signal
- KA:
-
Kaininc acid
- KO:
-
Knock-out
- ODCs:
-
Oligodendrocytes
- OPCs:
-
Oligodendrocyte-precursor cells
- ORT:
-
Object recognition test
- PDN:
-
Postnatal day
- PFC:
-
Prefrontal cortex
- PPI:
-
Protein–protein interaction
- PV+ :
-
Parvalbumin-expressing
- qPCR:
-
Quantitative PCR
- RNA-seq:
-
RNA sequencing
- sEPSCs:
-
Spontaneous excitatory postsynaptic currents
- snRNA-seq:
-
Single nucleus RNA sequencing
- WT:
-
Wild-type
- Zolp:
-
Zolpidem
References
van de Kamp JM, Mancini GM, Salomons GS (2014) X-linked creatine transporter deficiency: clinical aspects and pathophysiology. J Inherit Metab Dis 37:715–733
Joncquel-Chevalier Curt M, Voicu P-M, Fontaine M, Dessein A-F, Porchet N, Mention-Mulliez K et al (2015) Creatine biosynthesis and transport in health and disease. Biochimie 119:146–165
Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, Vorhees CV (2011) Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS ONE 6:e16187
Baroncelli L, Alessandrì MG, Tola J, Putignano E, Migliore M, Amendola E, et al. (2014): A novel mouse model of creatine transporter deficiency. F1000Res 3: 228.
Baroncelli L, Molinaro A, Cacciante F, Alessandrì MG, Napoli D, Putignano E et al (2016) A mouse model for creatine transporter deficiency reveals early onset cognitive impairment and neuropathology associated with brain aging. Hum Mol Genet 25:4186–4200
Stockebrand M, Sasani A, Das D, Hornig S, Hermans-Borgmeyer I, Lake HA et al (2018) A mouse model of creatine transporter deficiency reveals impaired motor function and muscle energy metabolism. Front Physiol 9:773
Duran-Trio L, Fernandes-Pires G, Simicic D, Grosse J, Roux-Petronelli C, Bruce SJ et al (2021) A new rat model of creatine transporter deficiency reveals behavioral disorder and altered brain metabolism. Sci Rep 11:1636
Ghirardini E, Calugi F, Sagona G, Di Vetta F, Palma M, Battini R et al (2021) The role of preclinical models in creatine transporter deficiency: neurobiological mechanisms biomarkers and therapeutic development. Genes. https://doi.org/10.3390/genes12081123
Perna MK, Kokenge AN, Miles KN, Udobi KC, Clark JF, Pyne-Geithman GJ et al (2016) Creatine transporter deficiency leads to increased whole body and cellular metabolism. Amino Acids 48:2057–2065
Giusti L, Molinaro A, Alessandrì MG, Boldrini C, Ciregia F, Lacerenza S et al (2019) Brain mitochondrial proteome alteration driven by creatine deficiency suggests novel therapeutic venues for creatine deficiency syndromes. Neuroscience 409:276–289
Abdulla ZI, Pennington JL, Gutierrez A, Skelton MR (2020) Creatine transporter knockout mice (Slc6a8) show increases in serotonin-related proteins and are resilient to learned helplessness. Behav Brain Res 377:112254
Chen H-R, Zhang-Brotzge X, Morozov YM, Li Y, Wang S, Zhang HH et al (2021) Creatine transporter deficiency impairs stress adaptation and brain energetics homeostasis. JCI Insight. https://doi.org/10.1172/jci.insight.140173
Wawro AM, Gajera CR, Baker SA, Nirschl JJ, Vogel H, Montine TJ (2021) Creatine transport and pathological changes in creatine transporter deficient mice. J Inherit Metab Dis 44:939–948
Molinaro A, Alessandrì MG, Putignano E, Leuzzi V, Cioni G, Baroncelli L, Pizzorusso T (2019) A nervous system-specific model of creatine transporter deficiency recapitulates the cognitive endophenotype of the disease: a longitudinal study. Sci Rep 9:62
Udobi KC, Kokenge AN, Hautman ER, Ullio G, Coene J, Williams MT et al (2018) Cognitive deficits and increases in creatine precursors in a brain-specific knockout of the creatine transporter gene Slc6a8. Genes Brain Behav 17:e12461
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86:883–901
Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75:762–777
Hyder F, Rothman DL, Bennett MR (2013) Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci U S A 110:3549–3554
Braissant O, Béard E, Torrent C, Henry H (2010) Dissociation of AGAT, GAMT and SLC6A8 in CNS: relevance to creatine deficiency syndromes. Neurobiol Dis 37:423–433
Lake BB, Ai R, Kaeser GE, Salathia NS, Yung YC, Liu R et al (2016) Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352:1586–1590
Yao Z, Liu H, **e F, Fischer S, Adkins RS, Aldridge AI et al (2021) A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 598:103–110
Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H et al (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174:1015-1030.e16
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47
Ge SX, Jung D, Yao R (2020) ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36:2628–2629
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S et al (2021) The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49:D605–D612
Cid E, Marquez-Galera A, Valero M, Gal B, Medeiros DC, Navarron CM et al (2021) Sublayer- and cell-type-specific neurodegenerative transcriptional trajectories in hippocampal sclerosis. Cell Rep 35:109229
Ting JT, Lee BR, Chong P, Soler-Llavina G, Cobbs C, Koch C et al (2018) Preparation of acute brain slices using an optimized n-methyl-d-glucamine protective recovery method. J Vis Exp. https://doi.org/10.3791/53825
Mazziotti R, Cacciante F, Sagona G, Lupori L, Gennaro M, Putignano E et al (2020) Novel translational phenotypes and biomarkers for creatine transporter deficiency. Brain Commun 2:fcaa089
Koopmans F, van Nierop P, Andres-Alonso M, Byrnes A, Cijsouw T, Coba MP et al (2019) SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron 103:217-234.e4
Monyer H, Markram H (2004) Interneuron diversity series: molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci 27:90–97
Hu H, Gan J, Jonas P (2014) Interneurons Fast-spiking, parvalbumin GABAergic interneurons: from cellular design to microcircuit function. Science 345:1255263
Carter BC, Bean BP (2009) Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron 64:898–909
Klausberger T, Roberts JDB, Somogyi P (2002) Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci 22:2513–2521
Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M et al (2002) Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol 451:103–110
Katagiri H, Fagiolini M, Hensch TK (2007) Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53:805–812
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40:1271–1296
Ellington WR (2001) Evolution and physiological roles of phosphagen systems. Annu Rev Physiol 63:289–325
Nabuurs CI, Choe CU, Veltien A, Kan HE, van Loon LJC, Rodenburg RJT et al (2013) Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J Physiol 591:571–592
Li S, Bianconi S, van der Veen JW, Dang Do A, Stolinski J, Cecil KM et al (2021) Oxidative phosphorylation in creatine transporter deficiency. NMR Biomed 34:e4419
Gorenberg EL, Chandra SS (2017) The role of co-chaperones in synaptic proteostasis and neurodegenerative disease. Front Neurosci 11:248
Li X, Wang C-Y (2021) From bulk, single-cell to spatial RNA sequencing. Int J Oral Sci 13:36
deGrauw TJ, Salomons GS, Cecil KM, Chuck G, Newmeyer A, Schapiro MB, Jakobs C (2002) Congenital creatine transporter deficiency. Neuropediatrics 33:232–238
van de Kamp JM, Betsalel OT, Mercimek-Mahmutoglu S, Abulhoul L, Grünewald S, Anselm I et al (2013) Phenotype and genotype in 101 males with X-linked creatine transporter deficiency. J Med Genet 50:463–472
Heussinger N, Saake M, Mennecke A, Dörr H-G, Trollmann R (2017) Variable white matter atrophy and intellectual development in a family with X-linked creatine transporter deficiency despite genotypic homogeneity. Pediatr Neurol 67:45–52
Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A (2011) Brain plasticity and disease: a matter of inhibition. Neural Plast 2011:286073
Wong-Riley MTT (2012) Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. Adv Exp Med Biol 748:283–304
Anderson TR, Huguenard JR, Prince DA (2010) Differential effects of Na+-K+ ATPase blockade on cortical layer V neurons. J Physiol 588:4401–4414
Murata K, Kinoshita T, Ishikawa T, Kuroda K, Hoshi M, Fukazawa Y (2020) Region- and neuronal-subtype-specific expression of Na, K-ATPase alpha and beta subunit isoforms in the mouse brain. J Comp Neurol 528:2654–2678
Smith RS, Florio M, Akula SK, Neil JE, Wang Y, Hill RS, et al (2021) Early role for a Na, K-ATPase in brain development. Proc Natl Acad Sci U S A https://doi.org/10.1073/pnas.2023333118
Ruden JB, Dugan LL, Konradi C (2021) Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology 46:279–287
Gulyás AI, Buzsáki G, Freund TF, Hirase H (2006) Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur J Neurosci 23:2581–2594
Kann O, Papageorgiou IE, Draguhn A (2014) Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab 34:1270–1282
Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO (2011) Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons. Brain 134:180
Scheuer T, Endesfelder S, Auf dem Brinke E, Bührer C, Schmitz T (2022) Neonatal oxidative stress impairs cortical synapse formation and GABA homeostasis in parvalbumin-expressing interneurons. Oxid Med Cell Longev 2022:8469756
Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM et al (2016) Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis 93:35–46
Patrizi A, Picard N, Simon AJ, Gunner G, Centofante E, Andrews NA, Fagiolini M (2016) Chronic administration of the N-Methyl-D-aspartate receptor antagonist ketamine improves rett syndrome phenotype. Biol Psychiatry 79:755–764
Mukherjee A, Carvalho F, Eliez S, Caroni P (2019) Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell 178:1387-1402.e14
de San Z, Martin J, Donato C, Peixoto J, Aguirre A, Choudhary V, De Stasi AM et al (2020) Alterations of specific cortical GABAergic circuits underlie abnormal network activity in a mouse model of Down syndrome. Elife. https://doi.org/10.7554/eLife.58731
Kalinowska M, van der Lei MB, Kitiashvili M, Mamcarz M, Oliveira MM, Longo F, Klann E (2022) Deletion of Fmr1 in parvalbumin-expressing neurons results in dysregulated translation and selective behavioral deficits associated with fragile X syndrome. Mol Autism 13:29
Braissant O, Henry H, Loup M, Eilers B, Bachmann C (2001) Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Brain Res Mol Brain Res 86:193–201
Baker SA, Gajera CR, Wawro AM, Corces MR, Montine TJ (2021) GATM and GAMT synthesize creatine locally throughout the mammalian body and within oligodendrocytes of the brain. Brain Res 1770:147627
Bonvento G, Valette J, Flament J, Mochel F, Brouillet E (2017) Imaging and spectroscopic approaches to probe brain energy metabolism dysregulation in neurodegenerative diseases. J Cereb Blood Flow Metab 37:1927–1943
Benamer N, Vidal M, Balia M, Angulo MC (2020) Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat Commun 11:5151
Dubey M, Pascual-Garcia M, Helmes K, Wever DD, Hamada MS, Kushner SA, Kole MHP (2022) Myelination synchronizes cortical oscillations by consolidating parvalbumin-mediated phasic inhibition. Elife. https://doi.org/10.7554/eLife.73827
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705
Auguste YSS, Ferro A, Kahng JA, Xavier AM, Dixon JR, Vrudhula U et al (2022) Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat Neurosci 25:1273–1278
Guedes JR, Ferreira PA, Costa JM, Cardoso AL, Peça J (2022) Microglia-dependent remodeling of neuronal circuits. J Neurochem 163:74–93
Olmos-Serrano JL, Corbin JG, Burns MP (2011) The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci 33:395–403
Acknowledgements
We thank Francesca Biondi for animal care, and Elena Novelli and M. Alejandro Expósito for technical support. We also thank Luca Salemmi for IT support. We are grateful for the assistance of A. Caler and the Instituto de Neurociencias Omics Facility with single-cell and sorting studies.
Funding
This work has been supported by grant GR-2017–02364378 funded by the Italian Ministry of Health and by Telethon grant GGP19177 to LB; Italian Ministry of Health, RC 2021; grant from Fondazione Cassa di Risparmio di Firenze “Human Brain Optical Map**” to TP; grants from the Spanish Ministry of Science and Innovation (MICINN) co-financed by ERDF (grant no. RTI2018-102260-B-I00; Generalitat Valenciana, project no. PROMETEO/2020/007; and CSIC Interdisciplinary Thematic Platform (PTI +) NEURO-AGINGl + (PTI-NEURO-AGING +). C.M.N-I. was the recipient of a FPI fellowship from the MICINN. The Instituto de Neurociencias (UMH-CSIC) is a “Centre of Excellence Severo Ochoa” (grant no. SEV-2017–0723).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by EG, GS, AMG, FC, CMN, FC, SC, FDV, LD, EP and LB. The first draft of the manuscript was written by LB and EG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments involving animals were authorized by the Italian Ministry of Health (#1052/2020-PR) and carried out in accordance with the European Directive of 22 September 2010 (EU/63/2010).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Supplementary Materials and Methods and Supplementary figures S1, S2, S3, S4, S5, S6, S7, S8.
Additional file 2:
Table S1. List of differentially expressed genes in bulk RNA-seq.
Additional file 3:
Table S2. Quantitative PCR validation of top differentially-expressed genes.
Additional file 4:
Table S3. Gene Ontology analysis for biological process for up- and downregulated genes in bulk RNA-seq.
Additional file 5:
Table S4. Gene Ontology analysis for cellular component for up- and downregulated genes in bulk RNA-seq.
Additional file 6:
Table S5. Clusters of protein-protein interactions (PPI) for the corresponding proteins of up- and downregulated genes in bulk RNA-seq.
Additional file 7:
Table S6. List of population marker genes used for supervised clustering analysis of snRNA-seq data.
Additional file 8:
Table S7. Quantification of cells belonging to each of the major population clusters in snRNA-seq data.
Additional file 9:
Table S8. List of differentially expressed genes in major sn-RNAseq clusters.
Additional file 10:
Table S9. Gene Ontology analysis for biological process for up- and downregulated genes in major sn-RNAseq clusters.
Additional file 11:
Table S10. Gene Ontology analysis for cellular component for up- and downregulated genes in major sn-RNAseq clusters.
Additional file 12:
Table S11. Number of reads for each biological replicate in bulk RNA-seq.
Additional file 13:
Table S12. Primer sequences for qPCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghirardini, E., Sagona, G., Marquez-Galera, A. et al. Cell-specific vulnerability to metabolic failure: the crucial role of parvalbumin expressing neurons in creatine transporter deficiency. acta neuropathol commun 11, 34 (2023). https://doi.org/10.1186/s40478-023-01533-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-023-01533-w