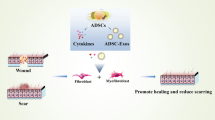
Abstract
Scar tissue is the inevitable result of repairing human skin after it has been subjected to external destructive stimuli. It leads to localized damage to the appearance of the skin, accompanied by symptoms such as itching and pain, which reduces the quality of life of the patient and causes serious medical burdens. With the continuous development of economy and society, there is an increasing demand for beauty. People are looking forward to a safer and more effective method to eliminate pathological scarring. In recent years, adipose-derived stem cells (ADSCs) have received increasing attention from researchers. It can effectively improve pathological scarring by mediating inflammation, regulating fibroblast proliferation and activation, and vascular reconstruction. This review focuses on the pathophysiological mechanisms of hypertrophic scarring, summarizing the therapeutic effects of in vitro, in vivo, and clinical studies on the therapeutic effects of ADSCs in the field of hypertrophic scarring prevention and treatment, the latest application techniques, such as cell-free therapies utilizing ADSCs, and discussing the advantages and limitations of ADSCs. Through this review, we hope to further understand the characterization of ADSC and clarify the effectiveness of its application in hypertrophic scarring treatment, so as to provide clinical guidance.
Similar content being viewed by others
Background
Scar tissue is an inevitable result of human skin repair after the exposure to destructive external stimuli, and is related to race, gender, age, and the tension, location, and pattern of injury of the wound [1]. If the injury reaches deeply into the reticular layer of the dermis, an abnormal fibroproliferative response occurs leading to hypertrophic scarring (HTS), which may even go on to develop into a keloid [2]. The characteristics of HTS are usually associated with collagen overproduction and an altered ratio of type I collagen to type III collagen [3, 4]. Approximately 35% of patients with postoperative scarring are estimated to develop HTS within one year [5]. Scarring not only leads to localized damage to the appearance of the skin, but also often accompanied by symptoms such as itching and pain [6]. Severe scar contracture located in the functional area may also cause mobility problems for the patient, which greatly reduces the quality of life and creates a burden on life and psychology [7].
The pathogenesis of HTS has not yet been clarified, and it is generally accepted that it may be related to the persistent inflammatory response and abnormal proliferation of fibroblasts, excessive vascular regeneration, and excessive extracellular matrix (ECM) deposition [3, 8]. HTS usually occurs in areas where the skin has been stretched, protrudes from the wound site, and grows rapidly over a period of 4–12 weeks, and once formed is difficult to recover as before [9, 10]. Clinical treatment of pathologic scarring is complex and difficult, and currently available treatments include localized compression, laser therapy, steroid injections, and surgical excision [11]. However, these methods cannot completely avoid excessive scar tissue formation, nor can they regenerate healthy dermal tissue, and may lead to skin ulcers, pain, localized tissue atrophy, hyperpigmentation or hypopigmentation, and many other complications [5, 12]. With the continuous development of economy and society, people’s demand for beauty is increasing, and they are looking forward to a safe and effective way to combat pathological scarring.
Adipose-derived stem cells (ADSCs) are a type of mesenchymal stem cells (MSCs) present in adipose tissue, and their function has received increasing attention from researchers in recent years due to their multi-differentiation potential, which allows them to differentiate into adipose and osteoblastic tissues [13, 14]. When skin trauma occurs, ADSCs and mature adipocytes mediate the inflammatory response and regulate the proliferation and activation of fibroblasts through paracrine secretion of a variety of cytokines, which has a certain therapeutic effect on dermal fibrosis and refractory scarring [15,16,17]. In addition, progressive reduction of intradermal adipocytes is a common pathology in many dermatofibrotic diseases [9, 18]. Last but not least, most patients would welcome the removal of some excess fat [19]. These findings suggest that ADSCs may play an important role in dermal fibrosis and scar formation, and therefore it is important to gain insight into the exact nature of the therapeutic effects of ADSCs on scarring. This article focuses on the pathophysiological mechanisms of skin scarring, summarizes the therapeutic effects of in vitro, in vivo, and clinical studies of ADSCs in the field of skin scarring prevention and treatment, the latest application techniques, and discusses the advantages and limitations of ADSCs with the aim of guiding the clinic. For this purpose, we searched PubMed, Web of Science, and EMBASE using “adipose-derived stem cells” or “ADSCs” as well as three related keywords. We primarily searched for original research published in the last 10 years on cellular studies, animal studies, and clinical trials on hypertrophic scarring.
Characterization of ADSCs
MSCs can be obtained from a variety of sites, including bone marrow, umbilical cord, and adipose tissue, but adipose tissue has emerged as the best source for harvesting MSCs [14]. Enzymatic digestion of adipose tissue produces many cells, including adipose-derived stem cells, preadipocytes, vascular endothelial cells, and fibroblasts, and this mixed cell population is known as stromal vascular fraction (SVF) [20]. ADSC was isolated from primary adipocytes by performing a passaging culture. In 2001, Zuk et al. first isolated ADSCs from the SVF of adipose tissue [21]. As a type of MSCs, ADSCs are more clinically attractive because they are easy to extract, less invasive, easy to obtain in large quantities, non-immunogenic, and do not involve ethical issues [14, 22]. It should be noted that ADSC has similar characteristics to bone marrow mesenchymal stem cells (BMSCs), but ADSC has a longer lifespan, higher proliferative capacity, shorter doubling time, and later in vitro senescence, which may be beneficial in the treatment of chronic or intractable diseases [23].
Sources
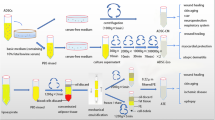
The abundant source of ADSCs is an important foundation for scar treatment research and application. First, with the improvement of living conditions, the rate of overweight and obesity has gradually increased, and liposuction and surgical removal of excess adipose tissue have made ADSCs more readily available [24]. Current methods of collecting ADSC include suction, mechanically assisted liposuction, power-assisted liposuction, laser-assisted liposuction, ultrasound-assisted liposuction, and surgical excision [14, 25]. Cells isolated by power-assisted techniques are characterized by high value-added potential and low senescence, and are therefore identified as the optimal approach [26]. Second, the isolation of ADSCs is relatively simple. In 1964, Rodbell described a method for isolating adipocytes from adipose tissue using collagenase, which became the “gold standard” [27]. Although trypsin, clostridial protease, and dispase are also used to isolate ADSCs, they may alter cell viability and reduce the regenerative potential of ADSCs [28]. Therefore, some scholars have attempted to use mechanical dissociation using scalpels or scissors, but the cell yield is low [29]. Finally, ADSC can proliferate stably in vitro, is easy to culture, and has a low mortality rate [29]. The whole extraction and culture process is shown in Fig. 1.
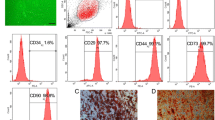
The process of harvesting, isolation, and characterization of ADSC. Adipose tissue was obtained by liposuction or surgical excision, digested by enzymes, and centrifuged to isolate the stromal vascular fraction. After collagenase digestion, red blood cell lysis, and washing, the stromal vascular fraction was cultured and analyzed by flow cytometry for the presence of cell surface markers to confirm the presence of ADSC characteristics. After culture, they are applied to the human body. Figure was drawn by the author
There are various types of adipose tissue in the human body, and ADSCs from different sexes and extraction sites have different proliferative, differentiation, paracrine, and anti-apoptotic capacities [30]. Subcutaneous adipose tissue is most commonly used for ADSC isolation because it can be obtained from the abdomen, thighs, and arms in a simple and non-invasive procedure [31]. Although it can also be extracted from areas such as the intrathoracic cavity, viscera, and retroperitoneum, studies have shown that ADSC from subcutaneous adipose tissue has a higher proliferative capacity and better anti-inflammatory effect [32]. In addition, tummy tucks are a popular site choice for surgeons because of their accessibility, abundance, and aesthetic improvement for patients. White adipose tissue is the source of ADSCs in most studies [29]. Brown adipose tissue has been thought to exist only in infants and to decrease with age [33]. However, recent studies have shown the presence of functional brown adipose tissue in adults with ADSC [32].
The age, weight, and disease state of the donor may affect the condition and nature of isolated ADSCs [34]. A study by Faustini et al. [35] showed that female adipose tissue obtained significantly higher ADSC production than male adipose tissue. In addition, ADSC obtained from obese donors showed increased proliferative and migratory capacity, excessive immune response, and reduced differentiation potential [36]. With aging, the proliferative and differentiation potential of acquired ADSCs is all over the place, and growth factor secretion becomes weaker [37, 38]. In addition, ADSCs provided by patients with diabetes, hypercholesterolemia, hypertension, and smoking were less pluripotent and self-renewing [31]. Platelet-rich plasma has been shown to improve human ADSC proliferation [39]. We believe it is necessary to use ADSCs isolated from healthy individuals to avoid poor efficacy and potential side effects.
Phenotypes
In recent years, scholars have been working to discover ADSCs-specific surface antigens [29]. In 2013, the International Society for Cell & Gene Therapy (ISCT) and the International Federation for Adipose Therapeutics and Science (IFATS) established that the minimum criteria to define ADSC must express CD73(+), CD90(+), CD105(+), and CD36(+) [40]. The expression of CD36 and lack of expression of CD106 distinguish ADSCs from BMSCs. Notably, the phenotype of ADSCs in culture is dynamic. For example, CD34 has been shown to be at its highest level in early ADSCs, while its expression decreases throughout the culture period [41]. There is some controversy about CD34, whose expression on ADSC is unstable [42,43,44]. Some authors have confirmed that cultured ADSC does not express CD34 [45], while others have reported that some fractions of ADSC are CD34(+) [44]. Comparison of sorted fractions from early passages of cultured human ADSC showed that CD34(+) cells have a greater proliferative potential and colony-forming capacity, whereas CD34(-) cells are characterized by a greater potential to differentiate into osteoblasts and adipocytes [46].
Adipose cell-free derivatives
ADSC exerts its therapeutic effects not only through direct cell-to-cell interactions, but also through the secretion of many molecules responsible for cell signaling, such as cytokines, growth factors, chemokines, extracellular vesicles, and other active substances [34, 47]. Cell-free therapies utilizing ADSC avoid the disadvantages of whole-cell drug delivery, such as potential tumorigenicity and storage issues [48]. Considering the effectiveness, safety, and cost aspects of ADSC, the dose and frequency of cell application cannot be increased indefinitely, therefore, ADSC-conditioned medium (ADSC-CM), ADSC-exosomes (ADSC-Exo), and other adipose cell-free rows of organisms have entered into the field of vision of researchers [49,50,51,52].
ADSC-CM is a wide-spectrum bioactive factor released by ADSC into the culture medium during cell culture and has been shown to promote wound healing and immunomodulation [53, 54]. ADSC-CM can reduce the cost of treatment and avoid the safety issues associated with stem cell therapy, but the short lifespan of the active ingredient, which is rapidly diluted and eliminated by diffusion, limits its use [16], as shown in Fig. 2. The normal wound healing process is transient, with most healing taking no more than 2 to 3 weeks. However, certain specific types of wounds, such as deep burns and infected wounds, form HTS characterized by extensive fibrosis due to prolonged healing time or the presence of a prolonged inflammatory response [18]. In molecular level, HTS has a large number of pro-fibrotic cytokines, inflammatory factors, etc. In histological level, it mainly consists of myofibroblasts (Myo-Fb) expressing α-smooth muscle actin (α-SMA) and ECM with predominantly type I collagen. It has a large number of inflammatory cells, immune cells, epidermal thickening with papilla reduction, and lacks normal skin appendages such as hair follicles, sweat glands, and sebaceous glands [62]. Changes in external tension and intracellular mechanical properties are associated with collagen, and scars are the specific result of collagen overproduction [63].
Normal healing process. Normal tissue repair involves many overlap** stages. After injury, hemostasis occurs initially through vasoconstriction and platelet aggregation. Subsequently, macrophages express inflammatory cytokines and chemokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-12, which recruit neutrophils and generate inflammation. Next, during the proliferative phase, macrophages promote tissue regeneration and ECM production by regulating the proliferation and migration of fibroblasts, and endothelial cells. As the tissue matures, neovascularization degenerates, and the ECM is rebuilt in the final remodeling phase. As granulation tissue is generated, fibroblasts differentiate into Myo-fibroblasts, which produce denser type I collagen and are responsible for wound contraction. Figure was drawn by the author
Inflammation
Inflammation consists of a vascular response (hemostasis) and a cellular response (inflammation) that lasts for the first 4 days after injury. After skin breakage, hemostasis is achieved by vasoconstriction, platelet aggregation, and activation of the coagulation cascade reaction to form a fibrin clot, preventing the loss of pathogens and body fluids [64]. Fibrin clots and damaged tissues release cytokines (TGF-β, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), FGF, and IL-8) to recruit neutrophils into the wound within 24–36 h [65]. Subsequently, neutrophils remove microbial pathogens by secreting proteases to digest damaged tissue [66].
Monocytes migrate to the wound, differentiate into macrophages, phagocytose apoptotic cells and cellular debris, and trigger an inflammatory response [67]. Interestingly, CD4+ T cells were associated with wound healing, while CD8+ T cells negatively affected this process [68]. M1 macrophages have pro-inflammatory properties and express inflammatory cytokines and chemokines, including TNF-α, IL-1β, and IL-12 [69, 70]. M2 macrophages secrete anti-inflammatory factors such as IL-10/TGF-β. Transformation from M1 to M2 macrophages contributes to the transition from the inflammatory phase to the proliferative phase [71, 72]. During abnormal wound healing, large numbers of macrophages inappropriately release cytokines between the late inflammatory and proliferative phases, promoting pathologic scarring.
Proliferation
The proliferative phase begins 3–4 days after injury and lasts 2–4 weeks. During the proliferative phase, a large number of new blood vessels and connective tissue are created, and the epithelium is re-formed [73, 74]. Reduces wound size through wound contraction and fibroproliferation. M2 macrophages promote tissue regeneration and ECM mass production by regulating the proliferation and migration of keratinocytes, fibroblasts, and endothelial cells [18, 75]. Fibroblasts begin to secrete large amounts of immature type III collagen into the matrix. As the tissue matures, neovascularization degenerates, and the ECM is rebuilt, entering the final remodeling phase [76].
Remodeling
Remodeling is the third stage of wound repair and is considered the most clinically important stage, beginning approximately 3 weeks after injury and lasting up to 2 years [77]. The cellular components that accumulate in the wound leave the site of injury and the vascularization gradually subsides, leaving a wound that heals without cellular collagen [78]. TGF-β regulatory factor stimulates fibroblasts to produce elastin and fibronectin, which ultimately form elastic fibers that give the skin a certain elasticity and participate in the restoration of the dermal structure [79]. Collagen overload from haphazard type III collagen to more effective and stronger type I collagen, increasing the strength of scar tissue [80]. Reaches plateau about 7 weeks after trauma [24, 81]. Subsequently, stimulated by growth factors, fibroblasts differentiate into Myo-Fb [82]. These cells are responsible for wound contraction and are characterized by the expression of α-SMA [83]. Myo-Fb contains bundles of microfilaments attached to extracellular fibronectin, and these microfilaments generate contractile forces around the ECM, causing the wound edges to contract by 0.75 mm per day, ultimately leading to wound closure [18]. It has been shown that the biological behavior of skin fibroblasts is influenced by skin tension during scar formation, showing stronger HTS changes at 10-15% stretch [83].
In physiologic wound healing, Myo-Fb undergoes apoptosis upon completion of the epithelialization process, which stops ECM deposition and wound contraction [84]. However, in HTS and keloids, there are features such as increased inflammatory response, overexpression of growth factors, increased activation and proliferation of fibroblasts, and massive neovascularization, which creates conditions for excessive collagen deposition in pathologic scars [10, 85]. The inability of fibroblasts to undergo apoptosis after the completion of epithelialization leads to cause granulation tissue contraction and secretion of a large number of dense, disordered collagen fibers, which leads to the formation of HTS [10]. In keloids, fibroblast proliferation is more pronounced and resistant to FAS-mediated apoptosis [86]. Therefore, inhibiting the inflammatory response, regulating the proliferation and activation of fibroblasts, and antagonizing the deposition of ECM and vascularization are the keys to treating pathological scarring [87].
Role of adipose-derived stem cells and secretome in scarring
Based on the full understanding of the pathophysiology of HTS formation, ADSCs have come into the limelight for their anti-inflammatory, immunomodulatory, fibrosis inhibiting, and vascular reconstruction effects [88], as shown in Fig. 3. A large number of studies have shown that ADSCs can be stimulated by the traumatic inflammatory environment, initiating immune regulation and attenuating the inflammatory response [7, 89]. At the same time, they secrete a variety of cytokines, inhibit TGF-β1 and collagen expression, promote matrix metalloproteinase (MMP) expression, accelerate ECM decomposition, inhibit fibrosis, and effectively improve the appearance, texture, thickness, and softness of the scar [90]. Paracrine cytokines, exosomes, and other active substances have been reported to be major factors in the exertion of the biological effects of ADSC [49]. ADSC-CM and ADSC-Exo have recently gained attention as alternatives to conventional ADSC therapy [91]. The related in vivo, in vitro studies are summarized in Table 1. However, as the acquisition of ADSCs not only requires additional collagenase for digestion, but also in vitro amplification and culture, its safety and long-term survival rate need to be examined [92].
Direct differentiation
ADSC has a multi-differentiation potential and under certain conditions can differentiate into adipocytes, muscle cells, osteoblasts, chondrocytes, vascular endothelial cells, etc. ADSC have also been shown to be able to differentiate into keratin-forming cells [93, 94]. These results suggest that ADSC may also differentiate directly into epidermal and dermal cells to promote tissue regeneration and prevent scar formation in injured areas during wound healing [94, 95]. When seeded on a synthetic or naturally-derived scaffold in vitro, stem cells can be differentiated toward a desired phenotype by the appropriate composition, appropriate structure, and appropriate physicochemical and mechanical properties of the scaffold [96,97,98,99].
Anti-inflammatory and immunomodulation
A moderate inflammatory response helps accelerate repair by removing inflammatory factors and cellular debris and fighting infection, whereas a chronic or excessive inflammatory response can lead to poor wound healing and pathologic scarring [100]. Therefore, surgeons emphasize modulating the inflammatory response in wounds to create an environment conducive to healing and reconstruction. It has been shown that ADSC upregulates inflammatory factors 2 weeks after injury (wound healing phase) and downregulates inflammatory factors 2 months after treatment (early scarring phase) [101].
After skin trauma, T cells, and macrophages persist, secreting and releasing a large number of inflammatory factors, especially IL-1, IL-6, and TNF-α, which aggravate the inflammatory response, leading to prolongation of the inflammatory phase and granulation proliferation to form scarring [102]. Xu et al. [103] found that ADSCs may greatly reduce scar formation during skin wound healing by modulating macrophage polarization. Liu et al. [104] found that ADSC-CM reduced the number of inflammatory cells in a keloid model and reduced angiogenesis and thus scar formation. In addition, insulin-like growth factor binding protein-7 secreted by ADSC inhibits the production of cytokines such as TGF-β1, VEGF, and IL-6 [105]. IL-10 is a potent anti-inflammatory cytokine, and in the context of pathologic scar formation, IL-10-modified ADSCs have been shown to have a beneficial effect on the proliferation, migration, and ECM synthesis of fibroblasts, accelerating wound healing time and decreasing scar area and scar prominence height [106]. In addition, ADSC-CM treatment promoted the M2 macrophage phenotype and induced the expression of IL-10 [107].
Activation of mast cells is a key factor in causing a chronic inflammatory response in scarring. Moderate amounts of mast cells contribute to wound healing; however, mast cells in HTS are 4 times larger than in normal skin, release inflammatory mediators histamine, IL-6, and IL-8, and promote inflammatory responses, inducing excessive ECM synthesis and vascularization in proliferative wounds, leading to HTS formation [108]. ADSC can further reduce proliferative scarring by inhibiting the number and activity of mast cells [94]. Prostaglandin E2 (PGE2) is produced with the help of the cyclooxygenase (COX)-1 or COX-2 and is the most abundant form of prostaglandin in the body [109]. It is well-known that PGE2 produced by ADSCs has an anti-inflammatory effect in the context of wound healing and pathologic scar formation, as it inhibits the proliferation and function of immune cells and induces macrophages to upregulate IL-10 expression. Yang et al. [110] using ADSC-CM cultured fibroblast model and in mouse keloid tissue found significantly increased levels of COX-2 and PGE2.
Inhibition of fibrosis
Fibroblast activation and function are important for wound healing, but in pathological scarring, activation of fibroblasts themselves may lead to fibroblast deposition and scar formation [9, 111]. In pathological scarring, increased fibroblasts secrete TGF-β1, which reduces the dependence of fibroblasts on external growth stimulators and maintains a strong proliferative ability [112]. In addition, TGF-β1 accelerates fibroblast activation to Myo-Fb and secretes α-SMA causing scar contracture [83, 113]. Imai et al. [114] found that ADSC-CM was effective in inhibiting keloid contracture. TGF-β3, a key regulator of scarless healing, reduces early ECM deposition and resists scar formation by regulating the migration of keratinocytes and dermal fibroblasts [115]. The study showed that a high ratio of TGF-β3/β1 promotes scarless healing similar to fetal trauma [116, 117].
ADSC-Exos can stimulate the reconstruction of ECM by regulating fibroblast differentiation and gene expression, thereby promoting wound healing and preventing scarring [61]. Li et al. [118] showed that ADSC-Exos attenuated the expression of Col1, Col3, α-SMA, and p-Smad2/p-Smad3 in fibroblasts and attenuated collagen production. Wang et al. [119] found that intravenous injection of ADSC-Exos blocked the differentiation of fibroblasts to Myo-Fb and increased the ratio of TGF-β3 to TGF-β1, reducing the size of the scar in the wounds of mice. Li et al. [120] found that ADSC-CM reduced the expression of Col1, Col3, and α-SMA in vitro. HTS tissues cultured with ADSC-CM exhibited thinner, well-arranged collagen. Zhang et al. [121] optimized ADSC-CM for in situ cross-linking with polysaccharide hydrogels to significantly improve the therapeutic effect of inhibiting scar proliferation. Interestingly, recent studies have shown that adipocytes regenerate from Myo-Fb as a plastic cell type that can be used to treat human scars [122].
A characteristic manifestation of pathological scarring is excessive collagen and ECM deposition [123, 124]. Fibroblasts synthesize and remodel the ECM primarily by synthesizing MMPs and MMP inhibitors [125]. Excessive deposition of ECM can lead to scarring if it is not absorbed and remodeled in time [126, 127]. In HTS, the ratio between type I collagen and type III collagen (6:1) was lower than that in keloid (17:1), but the ratio in normal skin was 5:1. Reduced levels of MMP1 and MMP2 expression, and elevated levels of tissue inhibitor of metalloproteinase (TIMP)1, and TIMP2 expression are important mechanisms in the formation of HTS [128]. Wang et al. [129] showed that the expression of TIMP1 and the deposition of Col1 in keloid tissue were significantly reduced after co-culture of keloid tissue with ADSC-CM in vitro. In addition, the number of CD31 and CD34 vessels was significantly reduced. Thus, ADSC-CM exerted an anti-scarring effect by regulating collagen degradation alleviating the abnormal deposition of collagen, and inhibiting keloid angiogenesis. Zhang et al. [130] injected either ADSCs or ADSCs-CM into rabbit ear lesions resulted in a more normal appearance of the scar, a more regular organization of collagen, and a decrease in the expression of α-SMA and type Ι collagen. In addition, HGF is an antifibrotic cytokine involved in scar control. In pathological scar tissues, HGF secreted by ADSCs inhibited TGF-β expression, decreased the Col1/Col3 ratio and TIMP1 levels, and upregulated MMP-1 expression [131].
Vascular reconstruction
During the wound healing phase, the rate of angiogenesis is at its peak during the proliferation phase, and the number of blood vessels decreases during the remodeling phase, when the anti-vascular factors gradually rise and the pro-vascular factors gradually degrade; once this balance is broken, it will lead to more capillary generation and promote scarring. Therefore, if angiogenesis can be inhibited under certain conditions, scarring will be reduced to a certain extent. Studies have shown that ADSC injection reverses the abnormal vascularization pattern of scar tissue and remodels the microvascular structure [132, 133]. Li et al. [127] using an ex vivo tissue explant model, and found that ADSC-Exos significantly suppressed COL production and disrupted the microvessel structure. Foubert et al. [101] found that treatment with ADSC in pigs promoted more normal collagen organization, lengthened elastic fiber length, and reduced vascularity. However, a large number of previous studies have come to the opposite conclusion that promoting intra-incisional vascularization attenuates pathologic scar formation, so extensive experiments are still needed to confirm the relationship between vascularization and scar formation [134, 135].
Clinical trials of ADSCs
The first clinical application of ADSC was published in 2004 for the treatment of cranial defects in children, with new bone formation and eventual healing of the defect after 3 months of follow-up [136]. Since then, cells or cell fractions of adipose tissue origin have been increasingly used in clinical practice, including nanofat, SVF, ADSCs, and stem cell secretome. The therapeutic potential of these cells has been widely explored in a variety of diseases, including COPD, diabetic ulcers, Crohn’s disease, and others [137]. Many recent clinical trials of ADSCs applied to scarring have been completed or are ongoing, and these studies have shown that ADSCs can significantly induce skin repair and improve scar appearance [138,139,140,141,142]. However, in the follow-up of Gal et al. [143], no significant benefit of autologous fat graft treatment was found. The related clinical trials are summarized in Table 2. How to ensure that they play an active role in the treatment of pathologic scarring is an issue that needs to be focused on in subsequent studies. Although ADSCs show great potential in wound healing, there are still some challenges such as safety issues, determination of the optimal application method, and evaluation of long-term efficacy.
Safety
The first issue to be addressed in the application of ADSCs in the clinic is safety. The immunoreactivity of animal-derived products during ADSC culture is a concern. The direct use of tissue aspirated by liposuction may be safer and more effective because it avoids in vitro manipulations that may alter the biological function of ADSCs and circumvent regulatory issues. In addition, there are concerns that transplantation of ADSCs may promote cancer cell proliferation, immune rejection, and treatment resistance through similar mechanisms that promote tissue regeneration by secreting growth factors, VEGF, and ECM proteins. Although the discussion on the safety of ADSCs is ongoing, there is no doubt that large randomized and controlled clinical trials are essential to reach a final safety recommendation.
Separation and extraction standards
The lack of uniform and effective isolation and extraction methods is also an important reason that hinders the translation of ADSCs to the clinic. ADSCs obtained from different species and anatomical regions exhibit different characteristics. Currently, studies comparing the efficacy of ADSCs extracted by various extraction methods have not yet appeared, and therefore no standardized method has been defined. The equipment, reagents, and extraction environments used by different scholars also vary, and yields vary widely. In addition, the automated equipment used to isolate ADSC is classified as a Class III medical device by the U.S. Food and Drug Administration (FDA) and cannot be approved for clinical applications. In addition, the presence of collagenase in injectable ADSC products makes it difficult to obtain approval from authorities such as the FDA. Therefore, to produce large quantities of clinical-grade ADSC at present, more extensive research is needed to establish uniform and effective separation and extraction standards that comply with the Dynamic Pharmaceutical Manufacturing Practice, which will make a significant contribution to the development of adipose tissue engineering.
Evaluation of treatment effects
Another challenge faced by ADSC-based therapies in clinical translation is the uncertainty of their clinical efficacy. In in vitro models, our findings have focused on studies with 2D cell culture systems, and these extensions typically ignore key features such as cell-cell and cell-extracellular cell matrix interactions, and tissue architecture. Animal models have been used in preclinical studies to evaluate the efficacy and safety of treatments, but there is a lack of standard animal models of scarring, and the differences in the mechanisms of scar formation between pigs and humans due to the high economic cost of pigs, the loose skin of rodents that shrinks rapidly after trauma, and the skin of the ear-free ear, which contains a cartilaginous layer, are not ideal animal models for the study of scarring.
Conclusions
The advantages of ADSCs include easy extraction, less invasive, easy to obtain in large quantities, non-immunogenic, and no ethical issues involved. It has shown promising anti-scarring therapeutic effects through direct differentiation, immunomodulation, anti-inflammatory, anti-fibrotic, and modulation of angiogenesis. ADSC-based therapies, which mainly consist of ADSC and SVF, have been widely studied and applied in clinical practice. Through paracrine mechanisms, such as ADSC-CM and ADSC-Exo, are becoming increasingly popular with fewer ethical and safety concerns. However, there is some discrepancy between basic research and clinical practice. To apply ADSCs in clinical practice for the treatment of scarring still needs to go for continuous improvement, such as determining the optimal extraction method, dosage, and duration of action of ADSCs on scarring to maximize their effective effects. A large number of experiments and data are still needed to elucidate that ADSCs are indeed effective in scar control. Future studies should focus on solving these problems and promoting the wide application of adipose-derived stem cell therapy in wound healing. In conclusion, the author believes that in the near future, with the continuous improvement of industry laws and regulations, we will eventually overcome the difficult problem of scarring and apply ADSC technology in more related fields.
Data availability
Not applicable.
Abbreviations
- ADSCs:
-
Adipose-derived stem cells
- ADSC-CM:
-
ADSC-conditioned medium
- ADSC-Exo:
-
ADSC-exosome
- α-SMA:
-
α-smooth muscle actin
- bFGF:
-
Basic fibroblast growth factor
- BMSC:
-
Bone marrow mesenchymal stem cell
- COX:
-
Cyclooxygenase
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia inducible factor 1α
- HTS:
-
Hypertrophic scarring
- IL:
-
Interleukin
- MMP:
-
Matrix metalloproteinase
- MSCs:
-
Mesenchymal stem cells
- Myo-Fb:
-
Myofibroblasts
- PDGF:
-
Platelet-derived growth factor
- PGE2:
-
Prostaglandin E2
- SVF:
-
Stromal vascular fraction
- TGF-β1:
-
Transforming growth factor-β1
- TIMP:
-
Tissue inhibitor of metalloproteinase
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
References
Chen H, Hou K, Wu Y, Liu Z. Use of adipose stem cells against hypertrophic scarring or keloid. Front Cell Dev Biol. 2021;9:823694.
Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, et al. Skin tissue regeneration for burn injury. Stem Cell Res Ther. 2019;10(1):94.
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3–17.
Chai CY, Song J, Tan Z, Tai IC, Zhang C, Sun S. Adipose tissue-derived stem cells inhibit hypertrophic scar (HS) fibrosis via p38/MAPK pathway. J Cell Biochem. 2019;120(3):4057–64.
Jones N. Scar tissue. Current opinion in otolaryngology & head and neck surgery. 2010;18(4):261–5.
Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci. 2014;29(6):751–7.
Yu LJ, Wang XJ, Long X. Advances in Mechanisms for Prevention and Treatment of Pathological Scars with mesenchymal stem cells. Zhongguo Yi Xue Ke Xue yuan xue bao. Acta Academiae Medicinae Sinicae. 2017;39(4):573–77.
Martin P. Wound healing–aiming for perfect skin regeneration. Sci (New York NY). 1997;276(5309):75–81.
Zhang M, Chen H, Qian H, Wang C. Characterization of the skin keloid microenvironment. Cell Communication Signaling: CCS. 2023;21(1):207.
Qu M, Song N, Chai G, Wu X, Liu W. Pathological niche environment transforms dermal stem cells to keloid stem cells: a hypothesis of keloid formation and development. Med Hypotheses. 2013;81(5):807–12.
Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2016;138(3 Suppl):s165–78.
Yates CC, Rodrigues M, Nuschke A, Johnson ZI, Whaley D, Stolz D, et al. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther. 2017;8(1):193.
Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini-Khah Z, Peymanfar Y, Ghalamkari S, et al. Adipose-derived stem cells for wound healing. J Cell Physiol. 2019;234(6):7903–14.
Argentati C, Morena F, Bazzucchi M, Armentano I, Emiliani C, Martino S. Adipose stem cell translational applications: from bench-to-Bedside. Int J Mol Sci. 2018;19(11).
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, et al. Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Volume 114. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2019. p. 108765.
Almalki SG. Adipose-derived mesenchymal stem cells and wound healing: potential clinical applications in wound repair. Saudi Med J. 2022;43(10):1075–86.
Hoerst K, van den Broek L, Sachse C, Klein O, von Fritschen U, Gibbs S, et al. Regenerative potential of adipocytes in hypertrophic scars is mediated by myofibroblast reprogramming. J Mol Med. 2019;97(6):761–75.
Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. 2021;30(1):132–45.
Masson JK. Lipectomy: the surgical removal of excess fat. Postgrad Med. 1962;32:481–8.
Zhou C, Zhang B, Yang Y, Jiang Q, Li T, Gong J, et al. Stem cell-derived exosomes: emerging therapeutic opportunities for wound healing. Stem Cell Res Ther. 2023;14(1):107.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Sheykhhasan M, Wong JKL, Seifalian AM. Human adipose-derived stem cells with great therapeutic potential. Curr Stem Cell Res Therapy. 2019;14(7):532–48.
Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel-Daim MM. Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Volume 107. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2018. pp. 625–33.
Zahorec P, Sarkozyova N, Ferancikova N, Bukovcan P, Danisovic L, Bohac M, et al. Autologous mesenchymal stem cells application in post-burn scars treatment: a preliminary study. Cell Tissue Banking. 2021;22(1):39–46.
Egro FM, Coleman SR. Facial Fat Grafting: the past, Present, and Future. Clin Plast Surg. 2020;47(1):1–6.
Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-derived stem cells for tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016;2016:6737345.
Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods (San Diego Calif). 2008;45(2):115–20.
Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, et al. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transpl. 2013;22(4):701–9.
Zou ML, Liu SY, Sun ZL, Wu JJ, Yuan ZD, Teng YY, et al. Insights into the role of adipose-derived stem cells: wound healing and clinical regenerative potential. J Cell Physiol. 2021;236(4):2290–97.
Fraser J, Wulur I, Alfonso Z, Zhu M, Wheeler E. Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy. 2007;9(5):459–67.
Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36(4):1111–26.
Ong WK, Chakraborty S, Sugii S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules. 2021;11(7).
Yang JP, Anderson AE, McCartney A, Ory X, Ma G, Pappalardo E, et al. Metabolically active three-Dimensional Brown Adipose tissue Engineered from White adipose-derived stem cells. Tissue Eng Part A. 2017;23(7–8):253–62.
Schneider I, Calcagni M, Buschmann J. Adipose-derived stem cells applied in skin diseases, wound healing and skin defects: a review. Cytotherapy. 2023;25(2):105–19.
Faustini M, Bucco M, Chlapanidas T, Lucconi G, Marazzi M, Tosca MC, et al. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng Part C Methods. 2010;16(6):1515–21.
Vyas KS, Bole M, Vasconez HC, Banuelos JM, Martinez-Jorge J, Tran N, et al. Profile of adipose-derived stem cells in obese and lean environments. Aesthetic Plast Surg. 2019;43(6):1635–45.
Hamel KM, Liimatta KQ, Belgodere JA, Bunnell BA, Gimble JM, Martin EC. Adipose-derived Stromal/Stem cell response to tumors and wounds: evaluation of patient age. Stem Cells Dev. 2022;31(19–20):579–92.
Marx C, Silveira MD, Beyer Nardi N. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 2015;24(7):803–13.
Tatsis D, Vasalou V, Kotidis E, Anestiadou E, Grivas I, Cheva A et al. The combined use of platelet-rich plasma and adipose-derived mesenchymal stem cells promotes Healing. A review of experimental models and future perspectives. Biomolecules. 2021;11(10).
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8.
Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J stem Cells. 2013;5(1):1–8.
Guo J, Hu H, Gorecka J, Bai H, He H, Assi R, et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am J Physiol Cell Physiol. 2018;315(6):C885–96.
Izadi R, Hejazi SH, Bahramikia S. Injection of stem cells derived from allogeneic adipose tissue, a new strategy for the treatment of diabetic wounds. J Diabetes Complicat. 2023;37(7):108496.
Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14(10):1159–63.
Ni X, Shan X, Xu L, Yu W, Zhang M, Lei C, et al. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res Ther. 2021;12(1):226.
Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18(8):1201–10.
Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213–9.
Nang’ole WF, Omu A, Ogeng’o JA, Agak GW. Do mesenchymal stem cells influence keloid recurrence? Stem cells and cloning: advances and applications. 2022;15:77–84.
Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11(1):312.
An YH, Kim DH, Lee EJ, Lee D, Park MJ, Ko J, et al. High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring Wound Healing Applications. Front Bioeng Biotechnol. 2021;9:681501.
Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24(5).
Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257.
Zhang B, Wu Y, Mori M, Yoshimura K. Adipose-derived stem cell conditioned medium and Wound Healing: a systematic review. Tissue Eng Part B Reviews. 2022;28(4):830–47.
Li J, Yin Y, Zou J, Zhang E, Li Q, Chen L, et al. The adipose-derived stem cell peptide ADSCP2 alleviates hypertrophic scar fibrosis via binding with pyruvate carboxylase and remodeling the metabolic landscape. Acta Physiologica (Oxford England). 2023;238(4):e14010.
Zhong Y, Zhang Y, Yu A, Zhang Z, Deng Z, **ong K, et al. Therapeutic role of exosomes and conditioned medium in keloid and hypertrophic scar and possible mechanisms. Front Physiol. 2023;14:1247734.
Lu Y, Wen H, Huang J, Liao P, Liao H, Tu J, et al. Extracellular vesicle-enclosed mir-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020;24(17):9590–604.
Zhu YZ, Hu X, Zhang J, Wang ZH, Wu S, Yi YY. Extracellular vesicles derived from human adipose-derived stem cell prevent the formation of hypertrophic scar in a rabbit model. Ann Plast Surg. 2020;84(5):602–07.
Montero-Vilchez T, Sierra-Sánchez Á, Sanchez-Diaz M, Quiñones-Vico MI, Sanabria-de-la-Torre R, Martinez-Lopez A, et al. Mesenchymal stromal cell-conditioned medium for skin diseases: a systematic review. Front Cell Dev Biol. 2021;9:654210.
Tiede S, Ernst N, Bayat A, Paus R, Tronnier V, Zechel C. Basic fibroblast growth factor: a potential new therapeutic tool for the treatment of hypertrophic and keloid scars. Annals Anat = Anatomischer Anzeiger: Official Organ Anatomische Gesellschaft. 2009;191(1):33–44.
Ma J, Yong L, Lei P, Li H, Fang Y, Wang L, et al. Advances in microRNA from adipose-derived mesenchymal stem cell-derived exosome: focusing on wound healing. J Mater Chem B. 2022;10(46):9565–77.
An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993.
Amjadian S, Moradi S, Mohammadi P. The emerging therapeutic targets for Scar Management: genetic and epigenetic landscapes. Skin Pharmacol Physiol. 2022;35(5):247–65.
Song H, Liu T, Wang W, Pang H, Zhou Z, Lv Y, et al. Tension enhances cell proliferation and collagen synthesis by upregulating expressions of integrin αvβ3 in human keloid-derived mesenchymal stem cells. Life Sci. 2019;219:272–82.
Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60(6):661–71.
Armour A, Scott PG, Tredget EE. Cellular and molecular pathology of HTS: basis for treatment. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15 Suppl 1:S6–17.
Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P, et al. The roles of inflammation in Keloid and hypertrophic scars. Front Immunol. 2020;11:603187.
Hong YK, Chang YH, Lin YC, Chen B, Guevara BEK, Hsu CK. Inflammation in Wound Healing and pathological scarring. Adv Wound care. 2023;12(5):288–300.
Zhu Z, Ding J, Tredget EE. The molecular basis of hypertrophic scars. Burns Trauma. 2016;4:2.
Zhou B, Gao Z, Liu W, Wu X, Wang W. Important role of mechanical microenvironment on macrophage dysfunction during keloid pathogenesis. Exp Dermatol. 2022;31(3):375–80.
Limandjaja GC, Waaijman T, Roffel S, Niessen FB, Gibbs S. Monocytes co-cultured with reconstructed keloid and normal skin models skew towards M2 macrophage phenotype. Arch Dermatol Res. 2019;311(8):615–27.
Direder M, Weiss T, Copic D, Vorstandlechner V, Laggner M, Pfisterer K, et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022;108:55–76.
Xu M, Fang S, **e A. Posttranscriptional control of PLOD1 in adipose-derived stem cells regulates scar formation through altering macrophage polarization. Annals Translational Med. 2021;9(20):1573.
Huang C, Ogawa R. The vascular involvement in soft tissue fibrosis-lessons learned from pathological scarring. Int J Mol Sci. 2020;21(7).
Tang M, Bian W, Cheng L, Zhang L, ** R, Wang W, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF–β/Smad and ERK signaling pathways. Int J Mol Med. 2018;41(3):1487–99.
Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18(11):921–33.
Bux S, Madaree A. Keloids show regional distribution of proliferative and degenerate connective tissue elements. Cells Tissues Organs. 2010;191(3):213–34.
Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Association: JCMA. 2018;81(2):94–101.
Rippa AL, Kalabusheva EP, Vorotelyak EA. Regeneration of Dermis: scarring and cells involved. Cells. 2019;8(6).
**e F, Teng L, Xu J, Lu J, Zhang C, Yang L, et al. Adipose-derived mesenchymal stem cells inhibit cell proliferation and migration and suppress extracellular matrix synthesis in hypertrophic-scar and keloid fibroblasts. Experimental Therapeutic Med. 2021;21(2):139.
Feng F, Liu M, Pan L, Wu J, Wang C, Yang L, et al. Biomechanical Regulatory Factors and therapeutic targets in Keloid Fibrosis. Front Pharmacol. 2022;13:906212.
Mony MP, Harmon KA, Hess R, Dorafshar AH, Shafikhani SH. Updated Rev Hypertrophic Scarring Cells. 2023;12(5).
Shin D, Minn KW. The effect of myofibroblast on contracture of hypertrophic scar. Plast Reconstr Surg. 2004;113(2):633–40.
Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Volume 362. New York, NY: Science; 2018. 6417.
Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regeneration: Official Publication Wound Healing Soc [and] Eur Tissue Repair Soc. 2011;19(Suppl 1):s10–5.
Čoma M, Fröhlichová L, Urban L, Zajíček R, Urban T, Szabo P et al. Molecular changes underlying hypertrophic scarring following Burns Involve specific deregulations at all Wound Healing stages (inflammation, proliferation and maturation). Int J Mol Sci. 2021;22(2).
**a Y, Wang Y, Shan M, Hao Y, Liu H, Chen Q, et al. Advances in the pathogenesis and clinical application prospects of tumor biomolecules in keloid. Burns Trauma. 2022;10:tkac025.
Coentro JQ, Pugliese E, Hanley G, Raghunath M, Zeugolis DI. Current and upcoming therapies to modulate skin scarring and fibrosis. Adv Drug Deliv Rev. 2019;146:37–59.
Bojanic C, To K, Hatoum A, Shea J, Seah KTM, Khan W, et al. Mesenchymal stem cell therapy in hypertrophic and keloid scars. Cell Tissue Res. 2021;383(3):915–30.
Gupta MK, Ajay AK. Fat on sale: role of adipose-derived stem cells as anti-fibrosis agent in regenerative medicine. Stem Cell Res Ther. 2015;6:233.
He X, Zhang J, Luo L, Shi J, Hu D. New Progress of adipose-derived stem cells in the therapy of hypertrophic scars. Curr Stem Cell Res Therapy. 2020;15(1):77–85.
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in Regenerative Medicine. Int J Mol Sci. 2017;18(9).
Li Q, Zhang C, Fu X. Will stem cells bring hope to pathological skin scar treatment? Cytotherapy. 2016;18(8):943–56.
**ong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, et al. Exosomes from adipose-derived stem cells: the emerging roles and applications in tissue regeneration of Plastic and Cosmetic surgery. Front Cell Dev Biol. 2020;8:574223.
Putri KT, Prasetyono TOH. A critical review on the potential role of adipose-derived stem cells for future treatment of hypertrophic scars. J Cosmet Dermatol. 2022;21(5):1913–19.
Joshi A, Xu Z, Ikegami Y, Yamane S, Tsurashima M, Ijima H. Co-culture of mesenchymal stem cells and human umbilical vein endothelial cells on heparinized polycaprolactone/gelatin co-spun nanofibers for improved endothelium remodeling. Int J Biol Macromol. 2020;151:186–92.
Huang Q, Zou Y, Arno MC, Chen S, Wang T, Gao J, et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev. 2017;46(20):6255–75.
Li N, Bai B, Zhang H, Zhang W, Tang S. Adipose stem cell secretion combined with biomaterials facilitates large-area wound healing. Regen Med. 2020;15(11):2311–23.
Ahmadian E, Eftekhari A, Janas D, Vahedi P. Nanofiber scaffolds based on extracellular matrix for articular cartilage engineering: a perspective. Nanotheranostics. 2023;7(1):61–9.
Shahi S, Dehghani F, Abdolahinia ED, Sharifi S, Ahmadian E, Gajdács M, et al. Effect of gelatinous spongy scaffold containing nano-hydroxyapatite on the induction of odontogenic activity of dental pulp stem cells. J King Saud Univ - Sci. 2022;34(8):102340.
Seo BF, Jung SN. The Immunomodulatory effects of mesenchymal stem cells in Prevention or treatment of excessive scars. Stem Cells Int. 2016;2016:6937976.
Foubert P, Zafra D, Liu M, Rajoria R, Gutierrez D, Tenenhaus M, et al. Autologous adipose-derived regenerative cell therapy modulates development of hypertrophic scarring in a red Duroc porcine model. Stem Cell Res Ther. 2017;8(1):261.
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134(10):2648–57.
Xu M, Fang S, Ma X. CD73(+) adipose-derived stem cells reduce scar formation through PLOD1. Annals Translational Med. 2022;10(2):66.
Liu J, Ren J, Su L, Cheng S, Zhou J, Ye X, et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns: J Int Soc Burn Injuries. 2018;44(2):370–85.
Liu F, Yu T, Liu J, Yang Q, Wu J, Ren J, et al. IGFBP-7 secreted by adipose-derived stem cells inhibits keloid formation via the BRAF/MEK/ERK signaling pathway. J Dermatol Sci. 2023;111(1):10–9.
**e F, Teng L, Lu J, Xu J, Zhang C, Yang L, et al. Interleukin-10-Modified adipose-derived mesenchymal stem cells prevent hypertrophic scar formation via regulating the Biological characteristics of fibroblasts and inflammation. Mediators Inflamm. 2022;2022:6368311.
Kruger MJ, Conradie MM, Conradie M, van de Vyver M. ADSC-conditioned media elicit an ex vivo anti-inflammatory macrophage response. J Mol Endocrinol. 2018;61(4):173–84.
Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol. 2013;22(8):507–10.
Ud-Din S, Bayat A. Controlling inflammation pre-emptively or at the Time of Cutaneous Injury optimises Outcome of skin scarring. Front Immunol. 2022;13:883239.
Yang J, Li S, He L, Chen M. Adipose-derived stem cells inhibit dermal fibroblast growth and induce apoptosis in keloids through the arachidonic acid-derived cyclooxygenase-2/prostaglandin E2 cascade by paracrine. Burns Trauma. 2021;9:tkab020.
Li S, Yang J, Sun J, Chen M. Adipose-derived mesenchymal stem cells alleviate hypertrophic scar by inhibiting Bioactivity and Inducing apoptosis in hypertrophic scar fibroblasts. Cells. 2022;11:24.
Chen J, Li Z, Huang Z, Liang L, Chen M. Chyle Fat-derived stem cells conditioned medium inhibits hypertrophic scar fibroblast activity. Ann Plast Surg. 2019;83(3):271–77.
Chu H, Wang Y, Wang X, Song X, Liu H, Li X. Effects of transplanted adipose derived stem cells on the expressions of α-SMA and DCN in fibroblasts of hypertrophic scar tissues in rabbit ears. Experimental Therapeutic Med. 2018;16(3):1729–34.
Imai Y, Mori N, Nihashi Y, Kumagai Y, Shibuya Y, Oshima J et al. Therapeutic potential of adipose stem cell-derived conditioned medium on scar contraction model. Biomedicines. 2022;10(10).
Occleston NL, Laverty HG, O’Kane S, Ferguson MW. Prevention and reduction of scarring in the skin by transforming growth factor beta 3 (TGFbeta3): from laboratory discovery to clinical pharmaceutical. J Biomaterials Sci Polym Ed. 2008;19(8):1047–63.
Hu MS, Rennert RC, McArdle A, Chung MT, Walmsley GG, Longaker MT, et al. The role of stem cells during Scarless skin Wound Healing. Adv Wound care. 2014;3(4):304–14.
Yang GP, Lim IJ, Phan TT, Lorenz HP, Longaker MT. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2003;11(6):411–8.
Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221.
Wang L, Hu L, Zhou X, **ong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):13321.
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7(1):102.
Zhang C, Wang T, Zhang L, Chen P, Tang S, Chen A, et al. Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res Ther. 2021;12(1):23.
Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, et al. Regeneration of fat cells from myofibroblasts during wound healing. Sci (New York NY). 2017;355(6326):748–52.
Griffin MF, Borrelli MR, Garcia JT, Januszyk M, King M, Lerbs T, et al. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci Transl Med. 2021;13(609):eabb3312.
Wu ZY, Zhang HJ, Zhou ZH, Li ZP, Liao SM, Wu ZY, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10(3):1046–56.
Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med. 2000;6(4):391–407.
Zhou J, Shen JY, Tao LE, Chen H. The inhibition of adipose-derived stem cells on the Invasion of keloid fibroblasts. Int J Med Sci. 2022;19(12):1796–805.
Li J, Li Z, Wang S, Bi J, Huo R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered. 2022;13(4):8515–25.
Domergue S, Bony C, Maumus M, Toupet K, Frouin E, Rigau V, et al. Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars. PLoS ONE. 2016;11(5):e0156161.
Wang X, Ma Y, Gao Z, Yang J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res Ther. 2018;9(1):40.
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6(1):145.
Ma J, Yan X, Lin Y, Tan Q. Hepatocyte growth factor secreted from human adipose-derived stem cells inhibits fibrosis in hypertrophic scar fibroblasts. Curr Mol Med. 2020;20(7):558–71.
Garza RM, Paik KJ, Chung MT, Duscher D, Gurtner GC, Longaker MT, et al. Studies in fat grafting: part III. Fat grafting irradiated tissue–improved skin quality and decreased fat graft retention. Plast Reconstr Surg. 2014;134(2):249–57.
Luan A, Duscher D, Whittam AJ, Paik KJ, Zielins ER, Brett EA, et al. Cell-assisted Lipotransfer improves volume Retention in Irradiated Recipient sites and rescues Radiation-Induced skin changes. Stem Cells. 2016;34(3):668–73.
Wu D, Liu X, ** Z. Adipose-derived mesenchymal stem cells-sourced exosomal microRNA-7846-3p suppresses proliferation and pro-angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J Cosmet Dermatol. 2023;22(8):2333–42.
Luo Y, Yi X, Liang T, Jiang S, He R, Hu Y, et al. Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model. Stem Cell Res Ther. 2019;10(1):279.
Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. Journal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial surgery. 2004;32(6):370–3.
Deptuła M, Brzezicka A, Skoniecka A, Zieliński J, Pikuła M. Adipose-derived stromal cells for nonhealing wounds: emerging opportunities and challenges. Med Res Rev. 2021;41(4):2130–71.
Abou Eitta RS, Ismail AA, Abdelmaksoud RA, Ghezlan NA, Mehanna RA. Evaluation of autologous adipose-derived stem cells vs. fractional carbon dioxide laser in the treatment of post acne scars: a split-face study. Int J Dermatol. 2019;58(10):1212–22.
Kwon H, Lee S, Kim J, Song SH. Efficacy and safety of stromal vascular fraction on scar revision surgery: a prospective study. J Dermatolog Treat. 2023;34(1):2171260.
Behrangi E, Moradi S, Ghassemi M, Goodarzi A, Hanifnia A, Zare S, et al. The investigation of the efficacy and safety of stromal vascular fraction in the treatment of nanofat-treated acne scar: a randomized blinded controlled clinical trial. Stem Cell Res Ther. 2022;13(1):298.
van Dongen JA, van Boxtel J, Uguten M, Brouwer LA, Vermeulen KM, Melenhorst WB, et al. Tissue stromal vascular fraction improves early scar Healing: a prospective Randomized Multicenter Clinical Trial. Aesthetic Surg J. 2022;42(7):Np477–88.
Kemaloğlu CA, Özyazgan İ, Gönen ZB. Immediate fat and nanofat-enriched fat grafting in breast reduction for scar management. J Plast Surg Hand Surg. 2021;55(3):173–80.
Gal S, Ramirez JI, Maguina P. Autologous fat grafting does not improve burn scar appearance: a prospective, randomized, double-blinded, placebo-controlled, pilot study. Burns: J Int Soc Burn Injuries. 2017;43(3):486–89.
Acknowledgements
We would like to thank all the participants in the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and the main idea of the work. MLW drafted the main text, tables, and figures. MM and MRZ supervised the work, and provided additional scientifc information. JYZ revised the tables and figures. JCL, MM and MRZ reviewed and revised the text. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, M., Zhao, J., Li, J. et al. Insights into the role of adipose-derived stem cells and secretome: potential biology and clinical applications in hypertrophic scarring. Stem Cell Res Ther 15, 137 (2024). https://doi.org/10.1186/s13287-024-03749-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03749-6