Abstract
Chronic liver disease is responsible for significant morbidity and mortality worldwide. Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) can fully visualise the liver and adjacent structures in the upper abdomen providing a reproducible assessment of the liver and biliary system and can detect features of portal hypertension. Subjective interpretation of CT and MRI in the assessment of liver parenchyma for early and advanced stages of fibrosis (pre-cirrhosis), as well as severity of portal hypertension, is limited. Quantitative and reproducible measurements of hepatic and splenic volumes have been shown to correlate with fibrosis staging, clinical outcomes, and mortality. In this review, we will explore the role of volumetric measurements in relation to diagnosis, assessment of severity and prediction of outcomes in chronic liver disease patients. We conclude that volumetric analysis of the liver and spleen can provide important information in such patients, has the potential to stratify patients’ stage of hepatic fibrosis and disease severity, and can provide critical prognostic information.
Critical relevance statement
This review highlights the role of volumetric measurements of the liver and spleen using CT and MRI in relation to diagnosis, assessment of severity, and prediction of outcomes in chronic liver disease patients.
Key Points
-
Volumetry of the liver and spleen using CT and MRI correlates with hepatic fibrosis stages and cirrhosis.
-
Volumetric measurements correlate with chronic liver disease outcomes.
-
Fully automated methods for volumetry are required for implementation into routine clinical practice.
Graphical Abstract

Similar content being viewed by others
Introduction
Chronic liver disease (CLD) is responsible for significant morbidity and mortality worldwide [1]. The spectrum of CLD ranges from early asymptomatic hepatic inflammation, injury and/or fibrosis, to end-stage liver disease with complications of cirrhosis, portal hypertension, decompensation, liver cancer and death. Globally, hepatitis B and hepatitis C remain the most common causes of cirrhosis [2]. While viral hepatitis and alcoholic liver disease have traditionally contributed to the majority of CLD cases in Western countries, non-alcoholic fatty liver disease (NAFLD) is now a rapidly growing contributor to the CLD burden [2, 3].
Diagnosis, prognostication, and management of CLD rely upon clinical assessment, blood-based biochemistry, serological and molecular testing, invasive (liver biopsy) and non-invasive tests (liver elastography and imaging). Ultrasound (US) is the modality of choice for initial assessment of the liver due to wide availability, reduced cost, lack of ionising radiation, and avoidance of intravenous contrast administration [4]. However, some limitations in US imaging, such as the inability to fully visualise and characterise the liver parenchyma and focal lesions (e.g., in obese patients) [5], have led to the use of other modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) [6, 7]. These modalities can fully visualise the liver and adjacent structures in the upper abdomen providing a reproducible assessment of the liver and biliary system and can detect features of portal hypertension such as splenomegaly, even in markedly obese patients, where US is often impractical.
CT and MRI scans are usually performed with an intravenous contrast agent, which is important for characterising focal liver lesions. However, contrast-enhanced imaging is also used for the assessment of the liver parenchyma and vasculature (portal vein patency and porto-systemic shunts). Morphological changes of cirrhosis on CT and MRI include atrophy of the right lobe and segment IV, hypertrophy of segments I–III, liver surface nodularity (LSN), an expanded gallbladder fossa, enlargement of periportal spaces at the porta hepatis and the right lobe posterior notch. The accuracy of such findings for diagnosing cirrhosis ranges from 70 to 90% [8]. However, knowledge of the accuracy of subjective interpretation of CT and MRI in the assessment of liver parenchyma for early and advanced stages of fibrosis (pre-cirrhosis) is limited [9]. In one study assessing subjective assessment on MRI, LSN had 80% accuracy in detecting significant fibrosis (F2 or greater) [10].
To overcome this limitation, quantitative morphology-based methods have been suggested including manual and semi-automated measurements using CT images. These include 2D measurements of caudate-to-right lobe ratios, portal vein and hepatic vein diameters and ratios, parenchymal enhancement pre- and post-contrast administration and subjective or semi-automated assessment of LSN [11,12,13]. Such methods require additional, often time-consuming, measurements by experienced readers.
Volumetric measurements of the liver and spleen have been suggested as markers of liver disease severity. Such measurements can be obtained from CT or MRI scans performed with or without intravenous contrast. Portal hypertension leads to an increase in the size of the spleen, while distinct changes in the liver parenchyma occur as the severity of CLD increases [14, 15]. Segmental volumetric changes occur in advanced fibrosis and cirrhosis with gradual enlargement of segments I–III and atrophy of segments IV–VIII. Such changes in overall spleen and liver volumes and segmental volumes of the liver can be measured with advanced visualisation software packages manually, semi-automatically and, more recently, completely automatically [16]. These measurements can be used as absolute values or to derive ratios such as the liver-to-spleen ratio (LSR).
Artificial intelligence (AI) is a broad field that includes various automated methods to analyse imaging and non-imaging data. Deep learning is a subfield of AI that involves the use of neural networks to perform such tasks as image segmentation. Such methods have been applied to CT and MRI images to perform volumetric segmentation of the liver and spleen with high accuracy [17]. Automating volumetric segmentation using AI methods allows for rapid assessment of large datasets and perhaps implementation into routine care in the future.
In this article, we will review the literature on volumetric assessment of the liver and spleen in CLD and discuss its application in clinical practice. We will start by reviewing how these measurements are obtained and occasionally adjusted. We will then explore the role of volumetric measurements in relation to diagnosis, assessment of severity, and prediction of outcomes in CLD patients.
Methods
A literature review was conducted and information from relevant studies is summarised hereafter.
Literature search methodology
A literature search was conducted using PubMed and Google Scholar databases from inception to July 2023. Search terminology included ((liver volume OR spleen volume) AND (liver disease OR liver fibrosis OR cirrhosis OR portal hypertension OR hepatocellular carcinoma (HCC)) AND (computed tomography OR CT OR magnetic resonance imaging OR MRI)). The first 100 results from each database results were screened with title and abstract review. Potentially relevant articles were reviewed in full text. A citation review of relevant articles’ references and citing articles was also performed. Additional relevant articles including review articles, letters and pictorial reviews were reviewed for relevant references.
CLD quantitative evaluation methods
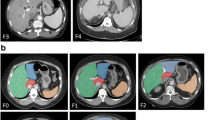
Volumetric analysis of liver and spleen size relied on manually contouring the margins of these organs on each slice of a CT or MRI scan, then multiplying the area measurements by slice thickness [18]. This is usually performed on axial slices (Fig. 1) but can also be performed using other planes such as coronal reformations of CT images or coronal MRI sequences. Such an approach is time-consuming and requires familiarity with imaging interpretation [19]. In addition, an understanding of the segmental anatomy of the liver (Couinaud segments) is required to accurately measure the volumes of hepatic segments (Fig. 1).
Developments in imaging software packages resulted in products that allow semi-automated segmentation of the liver and spleen which significantly improved volumetric analysis time [20, 21]. Furthermore, identifying anatomical landmarks within the liver within such packages allowed for accurate analysis of hepatic Couinaud segments. More recently, fully automated deep-learning approaches have been described for the segmentation of the liver and spleen with both excellent accuracy and speed [22]. Segmentation in this context refers to labelling of organs including the liver and spleen, rather than hepatic Couinaud segments. Such approaches rely on AI algorithms that have been trained on large datasets. Table 1 summarises the advantages and disadvantages of manual, semi-automated, and fully automated segmentation of the liver and spleen [19,20,21,22].
Estimation of liver and spleen volumes using two-dimensional measurements on CT or MRI has been shown to correlate accurately with volumetric analyses [23, 24]. Online calculators are available for liver and spleen volumetry using simple two-dimensional measurements (http://radclass.mudr.org/). Furthermore, stereological measurements of the liver have been successfully applied. Instead of measuring the whole area of an object on each axial plane and then summing them, stereological assessment employs statistical techniques such as a grid-based method which utilises liver pixel data on a grid placed over cross-sectional images. The pixels are created by a grid with intersecting parallel lines (usually horizontal and vertical) whereby the area of each pixel is known, and the number of pixels is used to estimate the areas and volumes occupied by an object. However, such methods still require additional software and are potentially influenced by slice thickness [25,26,27].
Absolute volumes, ratios and adjustments
Some studies used absolute volume measurements with cut-offs derived from earlier studies (Table 2) (Supplementary Fig. 1). The availability of volumetric data from large cohorts including diseased and healthy individuals allows for assessment of the normal range of organ volumes [22]. However, validation of normal ranges across different populations is required to standardise the measurements. Other studies utilise different adjustment parameters for the liver and spleen, due to individual variations in body size. Common approaches include using a ratio of liver-to-spleen known as the liver/spleen volume ratio (LSR) (Table 3) (Supplementary Fig. 2) or the liver segmental volume ratio (LSVR), a ratio of Couinaud segments I–III to IV–VIII [16, 28, 29]. Another approach has been to adjust the volumes of the liver or the spleen to body surface area (BSA) which relies on the height and weight of the individual or to adjust to body weight [30,31,32]. A few studies have incorporated platelet count or albumin into liver and splenic volumes [33,34,35,36,37,38].
Thresholds and normal ranges
While the current literature supports the use of volumetric measurements in CLD patients with available CT or MRI data, deriving useful thresholds suitable for clinical use remains challenging. Data from a healthy Korean cohort (2989 subjects) for normal spleen volume has been suggested by Kim and Ha (mean ± SD; men, 194.1 ± 64.2 cm3; women, 148.8 ± 47.0 cm3) [22]. However, the use of such thresholds in a different population becomes problematic where reported normal spleen volumes are potentially different due to ethnicity, age, or body habitus. This is evidenced by data from a large cohort from the United States of 8853 patients undergoing CT colonography or CT for renal donor work-up whereby the mean spleen volume for males and females was 216 ± 100 cm3 [39]. A summary of studies reporting normal spleen volumes is provided in Supplementary Table 7 [22, 24, 31, 39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. Such country-specific thresholds for normal splenic volume remain below the reported splenic volumes for CLD patients with no hepatic fibrosis (Table 2). Therefore, splenic volume thresholds for excluding hepatic fibrosis in CLD patients may still be considered abnormal compared to a healthy population with no CLD.
Similarly, a normal threshold for liver volume differs across different cohorts. The Korean study by Kim and Ha reported normal liver volumes (men, 1296.9 ± 212.5 cm3; women, 1058.0 ± 162.1 cm3) while a study from the United States of 3065 patients (from the same cohort above) [39] reported mean liver volume of 1533 ± 375 cm3. Using upper limits of normal with mean + 2 SD from such two cohorts, representing the largest cohorts in Asia and North America, respectively, will result in overlap** ranges for both spleen and liver volumes [22, 39]. Therefore, the use of sex-specific liver and spleen volumetric thresholds derived from local or similar reference cohorts is suggested where possible.
Spectrum of CLD, non-invasive tests and volumetric measurements
The spectrum of CLD includes pre-cirrhosis (fibrosis), compensated cirrhosis and decompensated cirrhosis with a higher risk of complications (organ failure, ascites, bleeding, and/or encephalopathy) as cirrhosis becomes decompensated. The accepted gold standard for fibrosis staging is liver biopsy which can be limited by sampling and interpretation errors [58,59,60]. Similarly, the accepted gold standard for the assessment of clinically significant portal hypertension is the hepatic venous pressure gradient (HVPG). These are invasive procedures that are associated with risks of bleeding, pain, hospitalisation and rarely death [61, 62]. Therefore non-invasive tests such as transient elastography, shearwave elastography, magnetic resonance elastography (MRE), and predictive scores such as aspartate aminotransferase (AST) to Platelet Ratio Index (APRI), Fibrosis-4 (FIB4), NAFLD fibrosis score, Fibroscan-AST (FAST) [63], and AI-supported systems [64, 65] have been validated to predict the severity of liver fibrosis and clinical outcomes across varying aetiologies of CLD. Clinico-pathological risk scores such as Child-Pugh class and model for end-stage liver disease (MELD) score are widely utilised to predict the prognosis of patients with CLD [66].
Volumetric measurements of the liver and spleen in patients who have already undergone CT or MRI can complement or potentially substitute non-invasive tests mentioned above, when unavailable. These measurements can provide relevant additional information on the severity of CLD and assist with the prediction of clinical outcomes without requiring invasive procedures. The value of such volumetric measurements is discussed below. However, these outcomes are interlinked and sometimes develop concomitantly at different rates throughout the spectrum of CLD.
Clinical considerations
Cirrhosis and fibrosis staging
Several studies using CT and MRI have shown that volumetric analysis of the liver and spleen can be used to differentiate between cirrhotic and non-cirrhotic livers (Supplementary Table 1) [22, 34, 36, 49, 51, 67,68,69]. Torres et al in 1986 reported on morphological volume changes of hepatic segments using CT images of 75 biopsy-proven cirrhotic and 50 control patients with no liver disease. They demonstrated that the caudate lobe and left lateral segments were larger and the right lobe was smaller in cirrhotic patients compared to controls [67]. Subsequent studies in different populations confirmed these findings and showed additional correlations with significant and advanced fibrosis compared to patients without fibrosis (Supplementary Table 2). Kim and Ha used an AI algorithm for liver and spleen segmentation to compare liver and spleen volumes as well as LSR among 158 viral hepatitis B patients with 2989 healthy controls (living donors) who had CT scans. They provided reference values for normal ranges from the healthy control cohort and showed that the liver was smaller, the spleen was larger and the LSR was smaller in viral hepatitis B patients (with and without cirrhosis) compared to controls [22]. Lee et al demonstrated that LSVR measured with an AI algorithm can diagnose significant fibrosis and cirrhosis with moderate accuracy [16]. In addition, Son et al demonstrated that an AI segmentation algorithm for spleen and liver volume measurements could be used to accurately diagnose significant and advanced fibrosis in a Korean cohort [70]. Studies that correlated volumetric measurements with fibrosis staging reported areas under the receiver operating characteristic curve (AUC) ranging from 0.63 to 0.94 (Supplementary Table 2) [12, 16, 28, 31, 32, 44, 53, 68, 70,71,72,73,74,75,76].
Clinical severity of liver disease and portal hypertension
Among patients with established cirrhosis, the increase in clinical severity of CLD by Child-Pugh class has been correlated with a decrease in liver volume (Supplementary Table 3) [34, 49, 77, 78]. Chen et al assessed 205 cirrhotic patients from viral hepatitis B and 40 healthy controls who underwent liver MRI with manual volumetric measurements of the spleen and right lobe of the liver to obtain a right lobe to spleen ratio [49]. They achieved moderate to high accuracy in distinguishing between Child-Pugh classes. In a subsequent analysis of the same cohort, the authors demonstrated that the splenic volume to platelet count ratio improved the accuracy of distinguishing between Child-Pugh classes compared to splenic volume alone [34].
Among patients with primary sclerosing cholangitis (PSC), clinical severity is often assessed with the Mayo risk score rather than Child-Pugh class (Supplementary Table 3). Idilman et al assessed patients who underwent liver MRI with MRE. Volumetric measurements showed moderate accuracy in predicting the high Mayo risk score group but were inferior to liver stiffness using MRE [79]. Khoshpouri analysed PSC patients using CT and MRI to obtain volumetric measurements. The left lobe to total liver volume ratio showed the best correlation to differentiate low and intermediate from high-risk Mayo scores [80]. Of note, none of the studies evaluating liver volumetry in PSC patients explored volumetry of diseased and non-diseased parts of the liver parenchyma.
Furthermore, liver and spleen volumes have been correlated in patients with cirrhosis with HVPG, the gold standard for assessing the severity of portal hypertension (Supplementary Table 3) [81,82,83,84,85,86,87,88]. Tseng et al derived a formula using albumin, AST, platelet count, and liver volume in 77 cirrhotic patients with CT to identify clinically significant portal hypertension defined as HVPG ≥ 10 mmHg [83]. Yan and Wu developed a model incorporating LSR with the size of varices on endoscopy to predict HVPG in cirrhotic patients with viral hepatitis B. Their model achieved excellent accuracy in predicting HVPG > 12 mmHg [84]. Finally, Romero-Cristobal and Clemente-Sanchez used a volume index, derived from multiplying splenic volume by LSVR obtained from CT, to predict HVPG in patients with HCC. The volume index was similar to non-invasive tests and predicted HVPG ≥ 10 mmHg with moderate accuracy in their cohort and in an external cohort [82].
Predicting outcomes
The correlation between the severity of liver disease (including the fibrosis stage) and the risk of decompensation is well recognised [89]. Unsurprisingly, liver and spleen volumes have also been correlated with the risk of decompensation, need for transplantation and mortality (Supplementary Table 4) [29, 90,91,92,93,94,95,96,97,98,99,100,101,102,103,104].
Patel et al evaluated liver volumes of 584 patients with cirrhosis using CT scans. During a median follow-up of 3.1 years, 19% underwent liver transplantation and 23% died. Liver volumes were larger for those who survived compared to those who were transplanted or died and this remained significant after adjustment for age and MELD score [100]. In a subsequent analysis of the same cohort, Patel et al assessed the value of splenic volume and LSR. They found a significant correlation between a larger spleen and transplantation and mortality, but the correlation was not significant when age and MELD score were incorporated into the analysis [55].
Yoo et al studied a Korean cohort with viral hepatitis B with splenic volumes measured semi-automatically on CT. After a median follow-up of 7.7 years, a larger spleen was associated with decompensation, mortality, and development of HCC [103]. In a larger Korean cohort of 1027 patients with hepatitis B cirrhosis, Kwon et al used an AI algorithm to measure liver and spleen volumes. They demonstrated that LSR was independently associated with decompensation and transplantation-free survival after adjusting for Child-Pugh and MELD scores [29]. Hu et al incorporated liver volume with age, prothrombin time, grade of encephalopathy, bilirubin and hepatitis B viral load in a model predicting 28-day mortality in patients with acute or chronic liver failure. Liver volume was measured on CT semi-automatically and adjusted to an estimated liver volume calculated from age, thoracic width on CT and race (Asian or Caucasian). Their model achieved an AUC of 0.906 which was higher than other models that did not incorporate liver volume measurements [93].
The change in liver and spleen volumes over time has been correlated with clinical outcomes (Supplementary Table 4) [92, 95, 104, 105]. Using liver and spleen segmentation from MRI scans obtained for HCC screening at baseline and at 1-year follow-up, Heo et al evaluated 280 patients with a median follow-up of 8.7 years. They demonstrated that LSR correlated with decompensation and that LSR change at 1 year was an independent predictor of liver-related death or transplantation [92]. Change in spleen length has been studied in PSC patients. Jung et al showed that change in spleen length, measured on US or MRI, was an independent predictor of clinical outcomes (transplantation and liver-related death) [106]. Khoshpouri et al performed volumetric measurements on CT and MRI for 89 PSC patients and demonstrated that a change in spleen volume or left lobe to total liver volume ratio predicted transplant-free survival with moderate accuracy [95]. Changes in liver and spleen volumes have also been studied in patients with viral hepatitis C [105, 107]. Haider et al demonstrated a reduction in spleen volume among patients pre and post-treatment with antiviral therapy and an increase in spleen volume among untreated patients [105].
Gastroesophageal varices
The presence and risk of bleeding of gastroesophageal varices represent an important part of the initial assessment and monitoring of CLD patients with portal hypertension [89]. Studies utilising volumetry for assessment of this specific outcome are summarised in Supplementary Table 5 [30, 33,34,35,36,37,38, 55, 57, 108,109,110,111,112,113,114]. Kim et al assessed CLD patients for the presence of varices and the development of variceal bleeding. In their study, the authors measured the liver volume index which was defined as the liver volume measured on CT divided by estimated liver volume from a formula based on BSA. The liver volume index was an independent predictor of large varices and variceal bleeding [30]. Wan et al used a ratio of the caudate lobe to total liver volumes measured on CTs of patients with endoscopy correlation. Their ratio was significantly different between patients with low-risk varices and high-risk varices. Furthermore, the ratio was an independent predictor of first variceal bleeding [112]. Chen et al used the right lobe of liver and spleen volumes adjusted to platelet count from MRIs in patients with cirrhosis from hepatitis B, to predict the presence of varices [34]. Similarly, Lee et al used the spleen volume to platelet ratio in patients with hepatitis B-related cirrhosis who underwent an endoscopy and reported a balanced cutoff of > 3.78 (sensitivity, 69.4%; specificity, 78.5%) and high sensitivity cutoff of > 1.63 (sensitivity, 100%; specificity, 38.9%) to detect high-risk varices [35].
Hepatocellular carcinoma-related outcomes
Predicting the development of HCC
Yoo et al studied patients with viral hepatitis B with splenic volumes measured on CT (see above). After a median follow-up of 7.7 years, a larger spleen was associated with the development of HCC. In their cohort, HCC occurred in 19.5% with estimated 1-year, 3-year, 5-year and 7-year cumulative incidence rates of HCC 0.5%, 8.2%, 12.2% and 17.2%, respectively. The authors derived a 532 mL threshold for splenic volume for predicting the development of HCC [103]. Lee et al studied a similar viral hepatitis B cohort from Korea with 429 patients who underwent at least one multiphase CT for HCC surveillance. In their study, a liver volume index was calculated from an estimated liver volume (derived from a formula based on BSA) divided by CT-measured liver volume. The liver volume index was significantly correlated with the development of HCC. The authors then proceeded to create a nomogram incorporating age, sex, presence of cirrhosis and liver volume index which showed significantly better performance in predicting HCC development compared to traditional risk scores with AUCs of 0.758 vs. 0.661–0.712, respectively [115]. The same group utilised the liver volume index in a cohort with hepatitis C and demonstrated similar findings [116] (Supplementary Table 6) [103, 115,116,117].
Predicting outcomes following HCC treatment
Liver volumetry including future remnant liver volume has become a standard of care in preoperative assessment of patients considered for hepatectomy for HCC [118]. In addition to absolute volume measurements [119], the function of the future remnant liver can be assessed with various types of functional imaging to minimise the risk of post-hepatectomy liver failure (PHLF) [118]. For example, using gadoxetic acid liver MRI can provide measurements of liver enhancement, from non-contrast and 20-min delayed post-contrast phases, to assess liver function for the prediction of PHLF [120]. Spleen volume, with and without adjustment to future remnant liver volume, BSA or platelets, has also been correlated with PHLF and survival [121,122,123,124,125,126,127,128,129]. The use of liver and spleen volumetry has also been studied in predicting outcomes following locoregional treatments such as thermal ablation, transarterial chemoembolisation and transarterial radioembolisation and following systemic therapy [130,131,132,133,134,135,136]. A detailed description of findings from these studies is beyond the scope of this review due to the differences and complexity of such treatments. However, a summary of relevant studies is provided in Supplementary Table 6.
Polycystic liver disease
Polycystic liver disease is the formation of multiple cysts (typically more than 20) which causes progressive liver enlargement [137, 138]. A detailed discussion of this unique condition is beyond the scope of this review.
Discussion
Liver and spleen volumetry from CT and MRI provides useful information in patients with CLD. The studies discussed in this review demonstrate that different volumetric parameters, either separately or in conjunction with other biomarkers, can provide important diagnostic and prognostic models for various CLD outcomes. However, some points should be noted from experience in the literature. Studies using absolute volume measurements have shown significant correlations with outcomes but may not be universally valid due to differences in body habitus across different ethnic or geographic populations. An absolute organ volumetric normal reference range derived from a cohort in Asia may not be applicable to populations in Africa or North America and vice versa. Therefore, standardising volume measurements of the liver or spleen to a body size parameter such as BSA or using organ ratios (e.g. LSR or LSVR) may allow a more universal assessment regardless of body size.
With a few exceptions, the appearances of the liver and spleen on imaging are relatively similar across different aetiologies of CLD when cirrhosis and clinically significant portal hypertension are already established. However, different aetiologies may lead to different morphological changes in the liver and spleen in the early stages of CLD [77, 96]. For example, such differences may impact the validity of volumetric cut-offs and ratios in classifying fibrosis stages. Lee et al derived LSVR from a hepatitis C cohort for the classification of hepatic fibrosis. However, the accuracy of derived thresholds for identifying significant fibrosis and cirrhosis declined when applied to an external cohort of mixed aetiology CLD patients [16]. Therefore, volumetric data from large cohorts including different aetiologies of CLD are required to derive useful thresholds for organ volumes and ratios.
The timeline of studies on this topic reflects advancement in radiologic software technology. Early studies utilised manual segmentation with manual contouring of the liver or spleen on each slice. A transition to using semi-automated segmentation of the liver and spleen is now available in several advanced visualisation software packages that allow faster segmentation. However, this requires sending the images from CT or MRI consoles or from a Picture Archiving and Communication System (PACS) into these packages followed by analysis by an experienced radiologist, or imaging technologist. These steps add to the complexity of incorporating volumetric analyses into routine practice and limit their use to select cases in specialised centres.
To overcome these issues, fully automated segmentation algorithms using AI deep-learning technology have been suggested. Studies using AI algorithms have shown accurate results in segmenting the liver and spleen without significant delays. Ahn et al developed a segmentation algorithm of the liver and spleen using labelled CT data with < 5% measurement error. They reported the time required for automated segmentation to be approximately 33 seconds per scan and the time required for review and correction to be < 1 min [17]. Subsequently, this algorithm was used on large-scale data ( > 3500 patients) in the study by Kim and Ha [22].
The transition to using AI algorithms for segmentation in clinical practice requires several clinical and technical steps. Testing the accuracy of AI algorithms on a local cohort with and without AI algorithm re-training is required. Deployment includes integration into PACS, a step that involves technical expertise, additional software platforms and potentially additional hardware [139, 140]. Despite AI research progress in organ segmentation in abdominal imaging over more than a decade, this integration has not happened on a large scale. Therefore, there are two main interlinked requirements that are essential in the process of using liver and spleen volumetric analysis for routine imaging of CLD patients: (1) accurate automated tools that can be easily integrated into the PACS environments of multiple vendors, ideally open-source or affordable; and (2) reference values for normal ranges derived from population-level cohorts. The latter is time-consuming to perform without the former. Regular auditing of AI algorithmic output is required to ensure results remain accurate and appropriate. Continuous AI training on new data (e.g., new types of scanners) is also required to ensure high accuracy of AI algorithms. Until these requirements are met, the use of volumetric analysis of the liver and spleen on a large scale will remain limited to research settings.
In addition to hepatic and splenic volumetric assessment, several other quantitative measurements can be obtained from multiphase contrast-enhanced CT and MRI. For example, liver parenchymal enhancement from non-contrast and equilibrium phases to derive extracellular volume fraction has been correlated with the severity of CLD [13, 141, 142]. Furthermore, the increased clinical use of gadoxetic acid liver MRI in CLD patients has led to the development of liver enhancement measurements which have been correlated with liver function, CLD severity, and postoperative complications in patients undergoing hepatectomy [78, 120, 143, 144].
Clinical context in liver and spleen volume measurements is important. The use of such volumetric analyses has been described in carefully selected CLD cohorts with some studies comparing CLD patients to normal controls. However, when automated organ volumetry is applied in clinical practice to routine abdominal CT and MRI scans, both CLD and non-CLD patients will be analysed. Liver and spleen volumes are influenced by various infective, inflammatory, and neoplastic pathophysiological processes in addition to CLD. Leveraging non-imaging data from the medical records to identify which patients would benefit from reporting liver and spleen volumes would require further integration and linkage of the different digital medical records platforms. Such additional data can also be part of multimodality models combining imaging and non-imaging data for the assessment of CLD patients.
In summary, volumetric analysis of the liver and spleen provides important information in patients with CLD who undergo CT or MRI imaging. These analyses have the potential to stratify patients’ stage of hepatic fibrosis and CLD severity, and provide prognostic information such as the risk of future decompensation, development of HCC and mortality. Fully automated AI segmentation tools have the potential to provide accurate, reproducible volumetric measurements without significant additional processing time. Solutions for the integration of such tools into clinical practice to allow large-scale applications are required.
Abbreviations
- AI:
-
Artificial intelligence
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the receiver operating characteristic curve
- BSA:
-
Body surface area
- CLD:
-
Chronic liver disease
- CT:
-
Computed tomography
- HCC:
-
Hepatocellular carcinoma
- HVPG:
-
Hepatic venous pressure gradient
- LSN:
-
Liver surface nodularity
- LSR:
-
Liver-to-spleen ratio
- LSVR:
-
Liver segmental volume ratio
- MELD:
-
Model for end-stage liver disease
- MRE:
-
Magnetic resonance elastography
- MRI:
-
Magnetic resonance imaging
- NAFLD:
-
Non-alcoholic fatty liver disease
- PACS:
-
Picture Archiving and Communication System
- PHLF:
-
Post-hepatectomy liver failure
- PSC:
-
Primary sclerosing cholangitis
- US:
-
Ultrasound
References
Cheemerla S, Balakrishnan M (2021) Global epidemiology of chronic liver disease. Clin Liver Dis 17:365
Huang DQ, Terrault NA, Tacke F et al (2023) Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol 20:388–398
Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM (2020) Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72:1605–1616
Gerstenmaier J, Gibson R (2014) Ultrasound in chronic liver disease. Insights Imaging 5:441–455
Schoenberger H, Chong N, Fetzer DT et al (2022) Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin Gastroenterol Hepatol 20:1561–9.e4
An JY, Peña MA, Cunha GM et al (2020) Abbreviated MRI for hepatocellular carcinoma screening and surveillance. Radiographics 40:1916–1931
Vernuccio F, Cannella R, Bartolotta TV, Galia M, Tang A, Brancatelli G (2021) Advances in liver US, CT, and MRI: moving toward the future. Eur Radiol Exp 5:52
Horowitz JM, Venkatesh SK, Ehman RL et al (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radio (NY) 42:2037–2053
Choi KJ, Jang JK, Lee SS et al (2018) Development and validation of a deep learning system for staging liver fibrosis by using contrast agent–enhanced CT images in the liver. Radiology 289:688–697
Venkatesh SK, Yin M, Takahashi N, Glockner JF, Talwalkar JA, Ehman RL (2015) Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging 40:766–775
Heo S, Kim DW, Choi SH, Kim SW, Jang JK (2022) Diagnostic performance of liver fibrosis assessment by quantification of liver surface nodularity on computed tomography and magnetic resonance imaging: systematic review and meta-analysis. Eur Radiol 32:3377–3387
Obmann VC, Mertineit N, Berzigotti A et al (2018) CT predicts liver fibrosis: Prospective evaluation of morphology-and attenuation-based quantitative scores in routine portal venous abdominal scans. PLoS One 13:e0199611
Zissen MH, Wang ZJ, Yee J, Aslam R, Monto A, Yeh BM (2013) Contrast-enhanced CT quantification of the hepatic fractional extracellular space: correlation with diffuse liver disease severity. AJR Am J Roentgenol 201:1204–1210
Li L, Duan M, Chen W et al (2017) The spleen in liver cirrhosis: revisiting an old enemy with novel targets. J Transl Med 15:111
Brancatelli G, Federle MP, Ambrosini R et al (2007) Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 61:57–69
Lee S, Elton DC, Yang AH et al (2022) Fully automated and explainable liver segmental volume ratio and spleen segmentation at CT for diagnosing cirrhosis. Radiol Artif Intell 4:e210268
Ahn Y, Yoon JS, Lee SS et al (2020) Deep learning algorithm for automated segmentation and volume measurement of the liver and spleen using portal venous phase computed tomography images. Korean J Radiol 21:987–997
Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M (1979) Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med 90:185–187
Chartrand G, Cresson T, Chav R, Gotra A, Tang A, DeGuise J (2014) editors. Semi-automated liver CT segmentation using Laplacian meshes. In: 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI), IEEE
Hermoye L, Laamari-Azjal I, Cao Z et al (2005) Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology 234:171–178
Suzuki K, Epstein ML, Kohlbrenner R et al (2011) Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol 197:W706
Kim DW, Ha J, Lee SS et al (2021) Population-based and personalized reference intervals for liver and spleen volumes in healthy individuals and those with viral hepatitis. Radiology 301:339–347
Muggli D, Müller M, Karlo C, Fornaro J, Marincek B, Frauenfelder T (2009) A simple method to approximate liver size on cross-sectional images using living liver models. Clin Radiol 64:682–689
Prassopoulos P, Daskalogiannaki M, Raissaki M, Hatjidakis A, Gourtsoyiannis N (1997) Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol 7:246–248
Gotra A, Sivakumaran L, Chartrand G et al (2017) Liver segmentation: indications, techniques and future directions. Insights Imaging 8:377–392
Sahin B, Ergur H (2006) Assessment of the optimum section thickness for the estimation of liver volume using magnetic resonance images: a stereological gold standard study. Eur J Radiol 57:96–101
Torkzad MR, Norén A, Kullberg J (2012) Stereology: a novel technique for rapid assessment of liver volume. Insights Imaging 3:387–393
Ouyang G-X, Zhang Y-M, Zhu S-L et al (2018) A novel, non-invasive model for diagnosing liver fibrosis stage in patients with hepatocellular carcinoma. Sci Rep 8:13074
Kwon JH, Lee SS, Yoon JS et al (2021) Liver-to-spleen volume ratio automatically measured on CT predicts decompensation in patients with B viral compensated cirrhosis. Korean J Radiol 22:1985
Kim BH, Chung JW, Lee CS et al (2019) Liver volume index predicts the risk of esophageal variceal hemorrhage in cirrhotic patients on propranolol prophylaxis. Korean J Intern Med 34:1233
Liu P, Li P, He W, Zhao L-Q (2009) Liver and spleen volume variations in patients with hepatic fibrosis. World J Gastroenterol 15:3298
Tago K, Tsukada J, Sudo N et al (2022) Comparison between CT volumetry and extracellular volume fraction using liver dynamic CT for the predictive ability of liver fibrosis in patients with hepatocellular carcinoma. Eur Radiol 32:7555–7565
Yu S, Chen W, Jiang Z (2021) Platelet count/spleen volume ratio has a good predictive value for esophageal varices in patients with hepatitis B liver cirrhosis. PLoS One 16:e0260774
Chen X-L, Chen T-W, Zhang X-M et al (2015) Platelet count combined with right liver volume and spleen volume measured by magnetic resonance imaging for identifying cirrhosis and esophageal varices. World J Gastroenterol 21:10184
Lee C-m, Lee SS, Choi W-M et al (2021) An index based on deep learning–measured spleen volume on CT for the assessment of high-risk varix in B-viral compensated cirrhosis. Eur Radiol 31:3355–3365
Li H, Chen T-W, Li Z-L et al (2015) Albumin and magnetic resonance imaging-liver volume to identify hepatitis B-related cirrhosis and esophageal varices. World J Gastroenterol 21:988
Karatzas A, Triantos C, Kalafateli M et al (2016) Multidetector computed tomography versus platelet/spleen diameter ratio as methods for the detection of gastroesophageal varices. Ann Gastroenterol 29:71
Min YW, Bae SY, Gwak G-Y et al (2012) A clinical predictor of varices and portal hypertensive gastropathy in patients with chronic liver disease. Clin Mol Hepatol 18:178
Perez AA, Noe-Kim V, Lubner MG et al (2023) Automated deep learning artificial intelligence tool for spleen segmentation on CT: defining volume-based thresholds for splenomegaly. AJR Am J Roentgenol 221:611–619
Kaneko J, Sugawara Y, Matsui Y, Ohkubo T, Makuuchi M (2002) Normal splenic volume in adults by computed tomography. Hepato-Gastroenterol 49:1726–1727
Geraghty E, Boone J, McGahan J, Jain K (2004) Normal organ volume assessment from abdominal CT. Abdom Imaging 29:482–490
Kaneko J, Sugawara Y, Matsui Y, Makuuchi M (2008) Spleen size of live donors for liver transplantation. Surg Radiologic Anat 30:515–518
Harris A, Kamishima T, Hao HY et al (2010) Splenic volume measurements on computed tomography utilizing automatically contouring software and its relationship with age, gender, and anthropometric parameters. Eur J Radiol 75:e97–e101
Li WX, Zhao XT, Chai WM et al (2010) Hepatitis B virus-induced liver fibrosis and cirrhosis: the value of liver and spleen volumetry with multi-detector spiral computed tomography. J Dig Dis 11:215–223
Lee J, Kim KW, Lee H et al (2012) Semiautomated spleen volumetry with diffusion‐weighted MR imaging. Magn Reson Med 68:305–310
Asghar A, Agrawal D, Yunus S, Sharma P, Zaidi S, Sinha A (2011) Standard splenic volume estimation in north Indian adult population: Using 3d reconstruction of abdominal CT scan images. Anat Res Int 2011:707325
Srisajjakul S, Prapaisilp P, Laorratkul N (2012) Normal splenic volume assessment on CT in 426 adults. Siriraj Med J 64:43–46
Caglar V, Alkoç OA, Uygur R, Serdaroglu O, Ozen O (2014) Determination of normal splenic volume in relation to age, gender and body habitus: a stereological study on computed tomography. Folia Morphol (Warsz) 73:331–338
Chen X-L, Chen T-W, Zhang X-M et al (2014) Quantitative assessment of the presence and severity of cirrhosis in patients with hepatitis B using right liver lobe volume and spleen size measured at magnetic resonance imaging. PLoS One 9:e89973
Cruz-Romero C, Agarwal S, Abujudeh HH, Thrall J, Hahn PF (2016) Spleen volume on CT and the effect of abdominal trauma. Emerg Radiol 23:315–323
Feng LM, Lei SJ, Zeng X et al (2017) The evaluation of non‐invasive multi‐slice spiral computed tomography‐based indices for the diagnosis and prognosis prediction of liver cirrhosis. J Dig Dis 18:472–479
Chhetri PK, Poudel S (2019) Normal splenic volume assessment on CT in Nepalese population. Ind J Basic Appl Med Res 8:7–12
Cheng YW, Chang YC, Chen YL, Chen RC, Chou CT (2019) Feasibility of measuring spleen stiffness with MR elastography and splenic volume to predict hepatic fibrosis stage. PLoS One 14:e0217876
Kumar G, Kumar V, Kumari M, Mishra H, Suruthi T (2021) Abdominal CT scan based estimation of splenic index and splenic volume in North Indian adults. J Evol Med Dent Sci 10:2749–2754
Patel M, Tann M, Liangpunsakul S (2021) CT-scan based liver and spleen volume measurement as a prognostic indicator for patients with cirrhosis. Am J Med Sci 362:252–259
Sahin NE, Oner Z, Oner S, Turan MK (2022) A study on the correlation between spleen volume estimated via cavalieri principle on computed tomography images with basic hemogram and biochemical blood parameters. Anat Cell Biol 55:40
Li J, Li J, Ji Q et al (2022) Nomogram based on spleen volume expansion rate predicts esophagogastric varices bleeding risk in patients with hepatitis B liver cirrhosis. Front Surg 9:1019952
Davison BA, Harrison SA, Cotter G et al (2020) Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 73:1322–1332
Rousselet MC, Michalak S, Dupré F et al (2005) Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 41:257–264
Chindamo MC, Nunes-Pannain VL, Araújo-Neto JM et al (2015) Intermediate fibrosis staging in hepatitis C: a problem not overcome by optimal samples or pathologists’ expertise. Ann Hepatol 14:652–657
Howlett DC, Drinkwater KJ, Lawrence D, Barter S, Nicholson T (2013) Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality. Radiology 266:226–235
Suk KT (2014) Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol 20:6
Lazarus JV, Castera L, Mark HE et al (2023) Real-world evidence on non-invasive tests and associated cut-offs used to assess fibrosis in routine clinical practice. JHEP Rep 5:100596
Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, Treeprasertsuk S (2021) Application of artificial intelligence in chronic liver diseases: a systematic review and meta-analysis. BMC Gastroenterol 21:1–16
Blanes-Vidal V, Lindvig KP, Thiele M, Nadimi ES, Krag A (2022) Artificial intelligence outperforms standard blood-based scores in identifying liver fibrosis patients in primary care. Sci Rep 12:2914
Peng Y, Qi X, Guo X (2016) Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine (Baltimore) 95:e2877
Torres WE, Whitmire LF, Gedgaudas-McClees K, Bernardino ME (1986) Computed tomography of hepatic morphologic changes in cirrhosis of the liver. J Comput Assist Tomogr 10:47–50
Hayashi T, Saitoh S, Fukuzawa K et al (2017) Noninvasive assessment of advanced fibrosis based on hepatic volume in patients with nonalcoholic fatty liver disease. Gut Liver 11:674
Furusato Hunt OM, Lubner MG, Ziemlewicz TJ, Muñoz Del Rio A, Pickhardt PJ (2016) The liver segmental volume ratio for noninvasive detection of cirrhosis: comparison with established linear and volumetric measures. J Comput Assist Tomogr 40:478–484
Son JH, Lee SS, Lee Y et al (2020) Assessment of liver fibrosis severity using computed tomography–based liver and spleen volumetric indices in patients with chronic liver disease. Eur Radiol 30:3486–3496
Lubner MG, Graffy PM, Said A et al (2021) Utility of multiparametric CT for identification of high-risk NAFLD. AJR Am J Roentgenol 216:659–668
Pickhardt PJ, Graffy PM, Said A et al (2019) Noninvasive multi-parametric CT staging of HCV-related liver fibrosis:: correlation with the histopathologic METAVIR fibrosis score. AJR Am J Roentgenol 212:547
Pickhardt PJ, Malecki K, Hunt OF et al (2017) Hepatosplenic volumetric assessment at MDCT for staging liver fibrosis. Eur Radiol 27:3060–3068
Goshima S, Kanematsu M, Watanabe H et al (2012) Gd-EOB-DTPA-enhanced MR imaging: prediction of hepatic fibrosis stages using liver contrast enhancement index and liver-to-spleen volumetric ratio. J Magn Reson Imaging 36:1148–1153
Lotan E, Raskin SP, Amitai MM et al (2017) The role of liver segment-to-spleen volume ratio in the staging of hepatic fibrosis in patients with hepatitis C virus infection. Isr Med Assoc J 19:251–256
Tarao K, Hoshino H, Motohashi I et al (1989) Changes in liver and spleen volume in alcoholic liver fibrosis of man. Hepatology 9:589–593
Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T (2016) Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol 89:20150896
Li C, Liu H, Wang J et al (2022) Multiparametric MRI combined with liver volume for quantitative evaluation of liver function in patients with cirrhosis. Diagn Inter Radiol 28:547–554
Idilman IS, Low HM, Bakhshi Z, Eaton J, Venkatesh SK (2020) Comparison of liver stiffness measurement with MRE and liver and spleen volumetry for prediction of disease severity and hepatic decompensation in patients with primary sclerosing cholangitis. Abdom Radiol (NY) 45:701–709
Khoshpouri P, Ameli S, Ghasabeh MA et al (2018) Correlation between quantitative liver and spleen volumes and disease severity in primary sclerosing cholangitis as determined by Mayo risk score. Eur J Radiol 108:254–260
Palaniyappan N, Cox E, Bradley C et al (2016) Non-invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol 65:1131–1139
Romero-Cristóbal M, Clemente-Sánchez A, Ramón E et al (2023) CT-derived liver and spleen volume accurately diagnose clinically significant portal hypertension in patients with hepatocellular carcinoma. JHEP Rep 5:100645
Tseng Y, Ma L, Luo T et al (2018) Non-invasive predictive model for hepatic venous pressure gradient based on a 3-dimensional computed tomography volume rendering technology. Exp Ther Med 15:3329–3335
Yan SP, Wu H, Wang GC, Chen Y, Zhang CQ, Zhu Q (2015) A new model combining the liver/spleen volume ratio and classification of varices predicts HVPG in hepatitis B patients with cirrhosis. Eur J Gastroenterol Hepatol 27:335–343
Iranmanesh P, Vazquez O, Terraz S et al (2014) Accurate computed tomography-based portal pressure assessment in patients with hepatocellular carcinoma. J Hepatol 60:969–974
Talakić E, Schaffellner S, Kniepeiss D et al (2017) CT perfusion imaging of the liver and the spleen in patients with cirrhosis: is there a correlation between perfusion and portal venous hypertension? Eur Radiol 27:4173–4180
Kennedy P, Stocker D, Carbonell G et al (2022) MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. Eur Radiol 32:8339–8349
Wagner M, Hectors S, Bane O et al (2018) Noninvasive prediction of portal pressure with MR elastography and DCE‐MRI of the liver and spleen: preliminary results. J Magn Reson imaging 48:1091–1103
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C (2022) Baveno VII - Renewing consensus in portal hypertension. J Hepatol 76:959–974
Blachar A, Federle MP, Brancatelli G (2001) Primary biliary cirrhosis: clinical, pathologic, and helical CT findings in 53 patients. Radiology 220:329–336
Eaton JE, Welle CL, Monahan H et al (2022) Comparative performance of quantitative and qualitative magnetic resonance imaging metrics in primary sclerosing cholangitis. Gastro Hep Adv 1:287–295
Heo S, Lee SS, Kim SY et al (2022) Prediction of decompensation and death in advanced chronic liver disease using deep learning analysis of gadoxetic acid-enhanced MRI. Korean J Radiol 23:1269–1280
Hu C, Jiang N, Zheng J et al (2022) Liver volume based prediction model for patients with hepatitis B virus-related acute-on-chronic liver failure. J Hepatobiliary Pancreat Sci 29:1253–1263
Khoshpouri P, Ghadimi M, Rezvani Habibabadi R et al (2020) Cross-sectional imaging in patients with primary sclerosing cholangitis: single time-point liver or spleen volume is associated with survival. Eur J Radiol 132:109331
Khoshpouri P, Hazhirkarzar B, Ameli S et al (2019) Quantitative spleen and liver volume changes predict survival of patients with primary sclerosing cholangitis. Clin Radiol 74:734.e13–.e20
Kim I, Jang YJ, Ryeom H et al (2012) Variation in hepatic segmental volume distribution according to different causes of liver cirrhosis: CT volumetric evaluation. J Comput Assist Tomogr 36:220–225
Liu J, Zhou C, Wang Y et al (2021) The combination of Child-Pugh score and quantitative CT-based spleen volume could predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. Abdom Radiol (NY) 46:3464–3470
Marinelli B, Kang M, Martini M et al (2019) Combination of active transfer learning and natural language processing to improve liver volumetry using surrogate metrics with deep learning. Radiol Artif Intell 1:e180019
Murata Y, Abe M, Hiasa Y et al (2008) Liver/spleen volume ratio as a predictor of prognosis in primary biliary cirrhosis. J Gastroenterol 43:632–636
Patel M, Puangsricharoen P, Arshad HMS et al (2019) Does providing routine liver volume assessment add value when performing CT surveillance in cirrhotic patients? Abdom Radiol (NY) 44:3263–3272
Schindler P, Riegel A, Görlich D et al (2021) Lower ratio of liver volume and body weight is a negative predictor of survival after transjugular intrahepatic portosystemic shunt. J Pers Med 11:903
Wackenthaler A, Molière S, Artzner T et al (2022) Pre-operative CT scan helps predict outcome after liver transplantation for acute-on-chronic grade 3 liver failure. Eur Radiol 32:12–21
Yoo J, Kim SW, Lee DH, Bae JS, Cho EJ (2021) Prognostic role of spleen volume measurement using computed tomography in patients with compensated chronic liver disease from hepatitis B viral infection. Eur Radiol 31:1432–1442
Lopera JE, Speeg KV, Young C et al (2014) Effect of liver volume in morbidity and mortality after elective transjugular intrahepatic portosystemic shunt. Gastrointest Interv 3:93–97
Haider L, Mandorfer M, Güngören Z et al (2018) Noninvasive monitoring of liver disease regression after hepatitis C eradication using gadoxetic acid-enhanced MRI. Contrast Media Mol Imaging 2018:8489709
Jung F, Cazzagon N, Vettorazzi E et al (2019) Rate of spleen length progression is a marker of outcome in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 17:2613–2615
Seko Y, Moriguchi M, Takahashi A et al (2020) The association between the platelet count and liver volume in compensated cirrhosis patients after the eradication of hepatitis C virus by direct-acting antivirals. Intern Med 59:1811–1817
Pham JT, Kalantari J, Ji C et al (2020) Quantitative CT predictors of portal venous intervention in uncontrolled variceal bleeding. AJR Am J Roentgenol 215:1247–1251
Tan BG, Tang Z, Ou J et al (2023) A novel model based on liver/spleen volumes and portal vein diameter on MRI to predict variceal bleeding in HBV cirrhosis. Eur Radiol 33:1378–1387
Tan BG, Yang LQ, Wu YP et al (2022) Combinations of liver lobe and spleen volumes obtained on magnetic resonance imaging to predict esophagogastric variceal bleeding in hepatitis B-related cirrhotic patients: a prospective cohort study. Medicine (Baltimore) 101:e30616
Wan S, He Y, Zhang X, Wei Y, Song B (2022) Quantitative measurements of esophageal varices using computed tomography for prediction of severe varices and the risk of bleeding: a preliminary study. Insights Imaging 13:47
Wan S, Wei Y, Zhang X, Yang C, Song B (2021) CT-derived quantitative liver volumetric parameters for prediction of severe esophageal varices and the risk of first variceal hemorrhage. Eur J Radiol 144:109984
Gaduputi V, Patel H, Sakam S et al (2015) Value of portal venous system radiological indices in predicting esophageal varices. Clin Exp Gastroenterol 8:89–93
Yang L-B, Zhao G, Tantai X-X et al (2022) Non-invasive model for predicting esophageal varices based on liver and spleen volume. World J Clin Cases 10:11743
Lee CS, Jung YJ, Kim SS et al (2018) Liver volume-based prediction model stratifies risks for hepatocellular carcinoma in chronic hepatitis B patients on surveillance. PLoS One 13:e0190261
Kang N, Chung JW, Jang ES, Jeong SH, Kim JW (2021) Computed tomography-measured liver volume predicts the risk of hepatocellular carcinoma development in chronic hepatitis C patients. Dig Dis Sci 66:4536–4544
Fang T, Long G, Mi X, Su W, Mo L, Zhou L (2022) Splenic volume, an easy-to-use predictor of HCC late recurrence for hcc patients after hepatectomy. Front Oncol 12:876668
Entezari P, Toskich BB, Kim E et al (2022) Promoting surgical resection through future liver remnant hypertrophy. Radiographics 42:2166–2183
Shirabe K, Shimada M, Gion T et al (1999) Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 188:304–309
Asenbaum U, Kaczirek K, Ba-Ssalamah A et al (2018) Post-hepatectomy liver failure after major hepatic surgery: not only size matters. Eur Radiol 28:4748–4756
Chuang YH, Ou HY, Lazo MZ et al (2018) Predicting post-hepatectomy liver failure by combined volumetric, functional MR image and laboratory analysis. Liver Int 38:868–874
Peng W, Li J-W, Zhang X-Y et al (2019) A novel model for predicting posthepatectomy liver failure in patients with hepatocellular carcinoma. PLoS One 14:e0219219
Hao C, JunYong M, LiQiang Q, GuoWen Y (2019) Value of spleen stiffness and spleen volume in predicting posthepatectomy liver failure in patients with hepatocellular carcinoma. J Clin Hepatol 35:2725
Lin J, Chi MH, Zhang X, Weng SG (2019) Correlation of postoperative splenic volume increase with prognosis of hepatocellular carcinoma after curative hepatectomy. Can J Surg 62:418–425
**ng Y, Liu ZR, Yu W, Zhang HY, Song MM (2021) Risk factors for post-hepatectomy liver failure in 80 patients. World J Clin Cases 9:1793–1802
Bae JS, Lee DH, Yoo J et al (2021) Association between spleen volume and the post-hepatectomy liver failure and overall survival of patients with hepatocellular carcinoma after resection. Eur Radiol 31:2461–2471
Meng XQ, Miao H, **a Y et al (2023) A nomogram for predicting post-hepatectomy liver failure in patients with hepatocellular carcinoma based on spleen-volume-to-platelet ratio. Asian J Surg 46:399–404
Fernández-Placencia R, Golse N, Cano L et al (2020) Spleen volumetry and liver transient elastography: predictors of persistent posthepatectomy decompensation in patients with hepatocellular carcinoma. Surgery 168:17–24
Ou J, Yu L, Wenjian W, Daoquan W, Qiang X (2015) Clinical significance of spleen–remnant liver volume ratio in hepatocellular carcinoma surgery. Indian J Surg 77:811–815
Wu WC, Chiou YY, Hung HH et al (2012) Prognostic significance of computed tomography scan-derived splenic volume in hepatocellular carcinoma treated with radiofrequency ablation. J Clin Gastroenterol 46:789–795
Elsawy AA, Dawoud MM, Elarabawy RA, Mohamed WS, Dawoud RM (2020) Role of residual liver volumetry and function in prediction of liver tolerability after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients: deriving a clinical decision support score. Egypt J Radiol Nucl Med 51:152
Zhang JW, Feng XY, Liu HQ et al (2010) CT volume measurement for prognostic evaluation of unresectable hepatocellular carcinoma after TACE. World J Gastroenterol 16:2038–2045
Timaran Montenegro DE, Torres Ramirez CA, Mateo CY, Govea Palma J, Quiñones JC, Orozco Vazquez JS (2020) CT-based hepatic residual volume and predictors of outcomes of patients with hepatocellular carcinoma unsuitable for surgical therapy undergoing transarterial chemoembolization. Acad Radiol 27:807–814
Dai HT, Chen B, Tang KY et al (2021) Prognostic value of splenic volume in hepatocellular carcinoma patients receiving transarterial chemoembolization. J Gastrointest Oncol 12:1141–1151
Müller L, Kloeckner R, Mähringer-Kunz A et al (2022) Fully automated AI-based splenic segmentation for predicting survival and estimating the risk of hepatic decompensation in TACE patients with HCC. Eur Radiol 32:6302–6313
Müller L, Gairing SJ, Kloeckner R et al (2022) Baseline splenic volume outweighs immuno-modulated size changes with regard to survival outcome in patients with hepatocellular carcinoma under immunotherapy. Cancers (Basel) 14:3574.
Temmerman F, Gevers T, Ho TA et al (2013) Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment Pharm Ther 38:397–406
van Aerts RMM, Kievit W, D’Agnolo HMA et al (2019) Lanreotide reduces liver growth in patients with autosomal dominant polycystic liver and kidney disease. Gastroenterology 157:481–91.e7
Strohm L, Hehakaya C, Ranschaert ER, Boon WPC, Moors EHM (2020) Implementation of artificial intelligence (AI) applications in radiology: hindering and facilitating factors. Eur Radiol 30:5525–5532
Wiggins WF, Magudia K, Schmidt TMS et al (2021) Imaging AI in practice: a demonstration of future workflow using integration standards. Radiol Artif Intell 3:e210152
Kupczyk P, Mesropyan N, Isaak A et al (2021) Quantitative MRI of the liver: evaluation of extracellular volume fraction and other quantitative parameters in comparison to MR elastography for the assessment of hepatopathy. Magn Reson Imaging 77:7–13
Yoon JH, Lee JM, Kim JH et al (2021) Hepatic fibrosis grading with extracellular volume fraction from iodine map** in spectral liver CT. Eur J Radiol 137:109604
Poetter-Lang S, Bastati N, Messner A et al (2020) Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom Radiol (NY) 45:3532–3544
Yoon JH, Lee JM, Kang H-J et al (2019) Quantitative assessment of liver function by using gadoxetic acid–enhanced MRI: hepatocyte uptake ratio. Radiology 290:125–133
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
N.K., W.C. and R.L. worked on the preparation, writing and editing of the manuscript. R.L., M.G., A.T. and G.E. provided supervision and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kutaiba, N., Chung, W., Goodwin, M. et al. The impact of hepatic and splenic volumetric assessment in imaging for chronic liver disease: a narrative review. Insights Imaging 15, 146 (2024). https://doi.org/10.1186/s13244-024-01727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01727-3