Abstract
Purpose
Nitrification and denitrification in soil are key components of the global nitrogen cycle. This study was conducted to investigate the effect of biochar application on soil nitrogen and bacterial diversity.
Methods
Pot experiments were conducted to investigate the effects of different biochar-based rates 0% (CK), 0.5% (BC1), 1.0% (BC2), 2.0% (BC3), and 4.0% (BC4) on soil nutrient and bacterial community diversity and composition.
Results
The results indicate that the total nitrogen (TN) and ammonium nitrogen (AN) contents in the soil increased by 4.7–32.3% and 8.3–101.5%, respectively. The microbial biomass nitrogen (MBN) content increased with increased amounts of biochar rate. The application of biochar also significantly changed the soil bacterial community composition. The copy number of 16S marker gene of related enzymes to the nitrification process in BC2 was reduced by 20.1%. However, the gene expressions of nitric oxide reductase and nitrous oxide reductase in BC3 increased by 16.4% and 16.0%, respectively, compared to those in CK. AN, nitrate nitrogen (NN), and NN/TN were the main factors affecting the structure of the soil bacterial community. In addition, the expressions of nitrite reductase, hydroxylamine, and nitric oxide reductase (cytochrome c) were also significantly correlated.
Conclusion
Therefore, the applied biochar improved soil nitrogen availability and which ultimately resulted in an environmental risk decrease by soil nitrogen release inhibition.
Similar content being viewed by others
Introduction
Since nitrogen (N) is the most limiting nutrient in the growth and development of crops, the world's consumption of nitrogen-based fertilizers is about 119.4 million tons, with an annual growth rate of 1.4%. The problem of low N use efficiency is a worldwide problem. According to statistics, the N use efficiency of China’s main food crops is 27.5%, showing a gradual decline (Yang et al. 2017). Due to the high amount of N fertilization, plants grown on dry land soils, the N utilization rate of vegetable crops is only about 10% (Liu et al. 2021). Excessive N application can result in high nitrate leaching and groundwater contamination. Reducing the use of N-based fertilizers, improving the N use efficiency, and reducing N loss and its impact on the environment, with the premise of ensuring food security, are critical goals that must be addressed by China and other countries worldwide.
In recent years, the use of biochar as a soil additive to increase soil N retention and reduce nutrient leaching has increased. Research regarding the residence time of biochar in the soil and its influence on the soil N cycle has amplified its potential for positive regulation of soil N activities among researchers (Wang et al. 2020). As an external input material, biochar is a solid carbon-rich organic material generated by heating biomass under low oxygen or anoxic conditions. Previous studies have demonstrated that biochar addition reduces N leaching. This can be attributed to the increases in the cation and anion exchange capacities (CEC, AEC) of the soil by the biochar material (Sika and Hardie 2014). Although biochar is mostly inert, its high surface area, porous nature, and ability to adsorb soluble nutrients provide a suitable habitat for soil microorganisms and can improve the physical and chemical properties of the soil. In addition, adding biochar to the soil may change the soil microbial community composition (Xu et al. 2014). For example, biochar enhances the effectiveness of ammonia N through the adsorption of ammonium nitrogen (NH4+). Doydora et al. (2011) found that a mixed application of acidic biomass charcoal and livestock and poultry compost into the soil reduced the soil NH3 loss by more than 50%. Adsorption experiments have also shown that biochar can adsorb NH4+ in the soil solution, reducing the loss of soil N and thereby reducing the risk of pollution to nearby water bodies (Chen et al. 2013).
Nitrification, the two-step conversion of ammonium (NH4+) to NO3− via nitrite (NO2−), is generally thought to play a critical role in the N cycle. Ammonia oxidation is considered to be the rate-limiting step of nitrification and is catalyzed by ammonia monooxygenase (AMO), which is encoded by the amoA gene from both archaea (AOA amoA) and bacteria (AOB amoA). The short-term application of biochar has been shown to significantly alter the microbial community structure in yellow-brown soil, significantly reduce the gene expressions of ammonia synthesis-related enzymes and the abundance of ammonia-oxidizing archaea in the fluvo-aquic soil, and inhibit the ammonia oxidation of the soil (Zhang et al. 2018). Based on the clustering of OTU analysis results, Alpha diversity (Shannon, Chao, Ace, Simpson and Coverage) and species community results at different classification levels were analyzed to determine the bacterial community. OTUs of bacteria were classified using the SILVA (Release128) database, and they were denominated at the domain, phylum, class, order, family, and genus levels (Yang et al. 2019). The 16S function prediction uses PICRUSt (a bioinformatics software package designed to predict metagenome functional content from marker gene (e.g., 16S rRNA) surveys and full genomes) to eliminate the influence of the copy number of 16S marker genes in the species genome and compares with KEGG to obtain metabolic information at each level of the metabolic pathway and the number copies of related enzymes (Langille et al. 2013). The Illumina MiSeq sequencing data were deposited in the Sequence Read Archive of the National Center for Biotechnology Information database (accession number: PRJNA752436).
Statistical analyses
Statistical analyses were performed using Statistical Product and Service Solutions 22.0 (SPSS Inc., Chicago, IL, USA). Significant differences were obtained by a one-way analysis of variance (ANOVA), with means compared using Duncan’s multiple range test (p<0.05). Principal Component Analysis (PCA) was used to compare the soil bacterial community composition between the different treatments. Redundancy analysis (RDA) and Monte Carlo permutation tests were conducted using Canoco 5.0.
Results
Soil N availability and microbial biomass
Different biochar application rates significantly affected soil N availability (Table 1). Compared with CK, biochar application increased the soil TN content by 4.7–32.3%. Soil TN in BC3 and BC4 increased significantly (p < 0.05) and were significantly (p < 0.05) higher than in BC1 and BC2. NN contents in BC1, BC2, BC3, and BC4 were 6.4%, 9.5%, 11.6%, and 12.5% lower, respectively, than in CK. AN contents in BC2, BC3, and BC4 were 57.4%, 58.9%, and 101.5% higher, respectively, than in CK. MBN and AN trends were consistent for all treatments.
Effects of biochar on soil microbial diversity and community structure
Microbial richness and diversity indices
We observed 74,1965 quality sequences, with an average of 22,752 sequences per sample. The average base length was 416 bp for the bacterial 16S rRNA. The coverage index of soil amended with biochar was 97%, indicating that the dataset included all sequences between V3 and V4 regions and that the sequence data volumes were reasonable (Fig. 1). The number of public OTUs processed by each treatment was 2039, 70.0%, 66.6%, 65.2%, 67.2%, and 66.0% of the total OTUs from CK, BC1, BC2, BC3, and BC4, respectively.
The alpha diversity of bacteria communities was positively affected by the application of biochar rates (Table 2), and biochar treatments significantly increased the Ace, Chao, and Shannon indices. Compared with CK, the Ace and Chao indices increased in BC1 were 11.1% and 11.5%, respectively. With increased biochar application, the Shannon index of the soil bacteria increased. In contrast, the biochar treatments significantly decreased the Simpson index related to CK. The Simpson indices in treatments BC3 and BC4 were significantly lower by 72% and 60%, respectively, than in CK.
Effects of biochar on soil bacterial community composition
Analyses based on the 16S rRNA data indicate that the main bacterial phyla in the soil samples were Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, and Bacteroidetes. Their total relative abundance was 81.60–84.93%. The relative abundances of Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, and Bacteroidetes were 28.78–32.26%, 24.92–32.67%, 5.96–10.84%, 4.98–8.97%, and 5.53–7.14%, respectively (Fig. 2). The relative abundance of Proteobacteria in BC3 was 4.4% higher than in CK. Compared to CK, the relative abundance of Proteobacteria was significantly reduced in BC4. The relative abundances of Chloroflexi and Acidobacteria increased significantly with increased biochar application. Compared to CK, BC4 increased the relative abundances of these two phyla by 79.0% and 61.9%, respectively.
Effect of biochar on the principal components of soil bacterial communities
A PCA was performed on the soil bacterial communities with regard to the different biochar application rates, from which two principal factors were extracted (Fig. 3). The total interpreted amount was 80.47%, of which PC1 and PC2 comprised 51.23% and 29.24%, respectively. The soil samples from CK were distributed in the negative areas of PC2. BC1, BC2, BC3, and BC4 gradually changed from the negative area to the positive area of PC2 and were mainly distributed in the positive area of PC2.
Effect of biochar on predictive functional profiling of bacterial communities related to soil nitrification and denitrification using 16S rRNA marker gene sequences
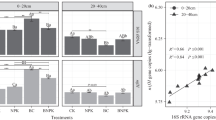
N cycling processes in the soil need to be coordinated by various enzymes in each branch (data URL: https://www.genome.jp/). According to the N metabolism pathway diagram, the corresponding relationship between the enzymes and genes related to N metabolism can be obtained. In addition, the enzymes involved in the soil nitrification and denitrification can be obtained by comparing sequence data to enzyme nomenclature (Table 3). We found that ammonia monooxygenase (1.14.99.39) and hydroxylamine dehydrogenase (1.7.2.6), which are involved in the ammonia oxidation process, were significantly different. Compared to CK, the ammonia oxygenase (1.14.99.39) in BC2 decreased by 20.1%, while BC3 and BC4 had increased gene expressions of 19.3% and 22.9%, respectively. In the N denitrification process, the copy number of 16S marker gene of related nitrite reductase, nitric oxide reductase nitrite reductase (1.7.2.1), nitric oxide reductase (1.7.2.5), and nitrous oxide reductase (1.7.2.4) in BC3 were 13.7%, 16.4%, and 16.0% greater, respectively, than in CK.
Correlations between soil bacterial community composition, soil N availability, and related enzyme gene expression
A redundant analysis (RDA) was used to analyze the correlations between soil N availability, the related enzyme functions, and the bacterial community composition. The first and second ordination axes explained 38.6% and 19.9% of the total variability, respectively (Fig. 4a). Regarding soil N availability, the main factors influencing the first ordination axis were NN (-0.5821), AN (0.5327), and NN/TN (-0.5312). Regarding enzymatic functions, the first and second ordination axes explained 26.5% and 24.9% of the total variability, respectively (Fig. 4b). The main factors influencing the first ordination axis were nitric oxide reductase (cytochrome c) (−0.5245), nitrous-oxide reductase (−0.2721), and ammonia monooxygenase (0.1272). The main factors influencing the second ordination axis were nitrite reductase (0.7263) and hydroxylamine (0.6929).
Discussion
Effect of biochar application rate on soil N availability
The soil N transformation process is an important part of the N cycle. Ammonium and nitrate N is a major factor determining plant growth and microorganisms (Sun et al. 2019). After biochar is applied to a field, it affects the transformation, migration, and distribution of soil N through its physical and chemical properties or by interacting with the soil (Li et al. 2020). The amount of biochar applied and the soil type are important factors that affect the migration, distribution, and leaching of soil N (Kumuduni et al. 2019). In this study, the application of biochar increased soil TN and AN contents by 4.7–32.3% and by 11.5–58.9%, respectively, indicating that the application of biochar can significantly increase the soil N content, mainly because the biochar has a rich pore structure and a large specific surface area, which can adsorb and hold soil N, reduce soil N leaching loss, and increase the soil N nutrient content (Abujabhah et al. 2018). Soil MBN is the most active component of soil organic N and plays an important role in regulating soil organic–inorganic N conversion and N cycling. Wardle et al. (2008) studied forest soils in northern Sweden and found that the addition of biochar promoted the growth of microorganisms, but Durenkamp et al. (2010) showed that the addition of biochar reduced the soil MBN content. The reason for this phenomenon is closely related to the test soil texture, original microbial biomass and nutrients, and the type of biochar. In this study, we found that the increase of biochar application rate increased MBN which can improve the availability of C in soil, thereby promoting the growth of microorganisms in the soil. In addition, although the application of biochar increased the soil TN and AN contents, it decreased the NN content by 2.6–12.5%. This is inconsistent with the results of Wang et al. (2012), in which the pot experiments showed that soil NN and AN contents increased significantly with an increased biochar application rate. The main reason for this is that the application of biochar loosens and ventilates the soil and transmits light, which is conducive to the growth of crop roots (Liu et al. 2021). We also found that the application of biochar increased the N absorption of the wheat roots by 15.3–65.2%.
Effect of biochar application rate on soil bacterial diversity and community composition
The changes of soil microbial community structure were affected by soil type, biochar type and biochar application amount (Dai et al. 2021). In this study, we found that with an increase in the amount of biochar applied, the diversity of the soil bacterial community initially increased, then decreased (Table 4). This is because increasing the amount of biochar will promote or inhibit the growth of certain types of bacteria, resulting in changes in the structure of the soil bacterial community (Zhang et al. 2017). In addition, the residence time of biochar in the soil will also affect the variations in the microbial community structure. In this study, with increased biochar application, the relative abundances of Chloroflexi and Actinobacteria increased, while the relative abundance of Acidobacteria decreased. In addition, addition of biochar increased the relative abundance of Nitrospirace, but decreased the relative abundances of Gemmatimonadetes and Patescibacteria. Acidobacteria mostly belong to the oligotrophic group, and the eutrophication state of the soil is not suitable for the growth of this group (Castro et al. 2013). The addition of biochar improves the nutrient status of the soil and increases the effective N content, thereby inhibiting the growth of Acidobacteria.
Nitrification is generally performed by ammonia-oxidizing bacteria and nitrite-oxidizing bacteria. Ammonia oxidation is the first and rate-limiting step of the nitrification process, which is mainly promoted by ammonia-oxidizing microorganisms (Yao et al. 2017). In this study, predictive functional profiling of bacterial communities related to soil nitrification and denitrification using 16S rRNA marker gene sequences. We found that, compared to CK, the number of copies of ammonia monooxygenase and hydroxylamine dehydrogenase in BC2 were significantly reduced, indicating that small amounts of biochar application had an impact on the growth and reproduction of AOA and AOB. In the nitrification process, the study of the 16S rRNA AOB gene sequence shows that AOB is mainly divided into the Proteus β subgroup and γ subgroup Nitrosospira (Shen et al. 2008). Studies have also shown that a “complete nitrifying bacteria” of the genus Nitrospirillum can directly oxidize NH3 to NO3-. This strain has functional genes encoding the ammonia oxidation and nitrite oxidation processes. However, the relative abundance of Nitrosospira in BC2 had little effect, indicating that it can inhibit the nitrite oxidation process, reduce the nitrification potential, and reduce nitrate leaching loss.
Denitrification is an important link that affects the global N cycle. According to the results, the number of copies of nitrification-related enzymes in BC3 and BC4 increased by 19.3% and 22.9%, respectively. It shows that high biochar application rate stimulates nitrification. This is consistent with the findings of Ball et al. (2010). Due to the porous characteristics of biochar, it provides a good environment for microorganisms, protects beneficial soil microorganisms, and accelerates the soil nitrification process. However, biochar also adsorbs phenolic compounds in the soil and inhibits the growth of nitrifying bacteria, thereby indirectly promoting soil nitrification. Studies have shown that adding biochar to farmland soil can improve soil aeration, inhibit the denitrification of anaerobic denitrifying microorganisms, and reduce nitrous oxide emissions. Harter et al. (2016) found that in slightly alkaline sandy soils, although biochar addition stimulated denitrification gene expression and increased denitrification, this is because biochar adsorbs nitrous oxide in the soil pores under water saturation. Cayuela et al. (2013) found that biochar generally reduces the proportion of nitrous oxide emissions in 15 different agricultural soils, indicating that biochar stimulates the final step of denitrification to reduce nitrous oxide to N. The results of this study, nitric oxide reductase (cytochrome c) and nitrous oxide reduction during the denitrification process increased in BC3 and BC4 by 16.4% and 16.0%, respectively (p<0.05), compared to CK. This result is consistent with previous results, indicating that the addition of biochar could promote the expression of denitrification genes and increases denitrification. This may be because the addition of biochar stimulates the bacterial nitrous oxide reductase activity encoded by nosZ and other reducing agents to reduce nitrous oxide (Harter et al. 2016).
Mechanism analysis of the effectiveness of biochar in regulating soil N
The soil microenvironment is closely related to the growth of soil microorganisms. Changes in soil nutrients, moisture, aeration, and other properties can cause changes in the composition and structure of soil bacterial communities (Zhou et al. 2019). The RDA conducted in this study found that AN, NN, and NN/TN in the soil were the main factors affecting of the soil bacterial community. In addition, the number of copies of the nitrite reductase, hydroxylamine, and nitric oxide reductase (cytochrome c) genes was also correlated with the abundance of some bacteria involved in the N cycle. Therefore, this study explored and inferred the biochar regulation mechanism on soil N content (Fig. 5): a low biochar application rate can improve the availability of N, which is mainly through reducing the expressions of ammonia monooxygenases and hydroxylamine dehydrogenase genes involved in the ammonia oxidation process, and affects the growth and reproduction rate of AOB in the soil, thereby inhibiting ammonia oxidation and a high biochar application rate can also improve the N utilization efficiency, mainly by increasing the expressions of nitric oxide reductase and nitrous oxide reductase in the denitrification process, stimulating the process of reducing N2O and NO to N2 during the last two steps of the denitrification process (Cayuela et al. 2013). The 16S function prediction level did not fully represent the activity of the soil microorganisms.
Therefore, biochar improved the availability of soil N and promoted the absorption of N by crops, mainly increasing the AN and NN contents. A low application rate biochar mainly inhibits the ammonia oxidation process, and a high application rate may improve soil N utilization efficiency by promoting nitrification and denitrification processes and which ultimately resulted in environmental risk decrease by soil nitrogen release inhibition.
Availability of data and materials
The Illumina MiSeq sequencing data were deposited in the Sequence Read Archive of the National Center for Biotechnology Information database (accession number: PRJNA752436)
Abbreviations
- AOA:
-
Ammonia-oxidizing archaea
- AOB:
-
Ammonia-oxidizing bacteria
- TN:
-
Total inorganic N
- NN:
-
Nitrate nitrogen
- AN:
-
Ammonium nitrogen
- MBN:
-
Microbial biomass
- OUT:
-
Operational taxonomic units
- AMO:
-
Ammonia monooxygenase
- Hao:
-
Hydroxylamine oxidoreductase
- NOR:
-
Nitrite oxidoreductase
- Nar:
-
Nitrate reductase
- Nir:
-
Nitrite reductase
- Nor:
-
Nitric oxide reductase
- Nos:
-
Nitrous oxide reductase
References
Abujabhah IS, Doyle RB, Bound SA et al (2018) Assessment of bacterial community composition, methanotrophic and nitrogen-cycling bacteria in three soils with different biochar application rates. J Soil Sediment 18:148–158
Ball PN, Mackenzie MD, Deluca TH et al (2010) Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J Environ Qual 39:1243–1253
Castaldi S, Riondino M, Baronti S et al (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85:1464–1471
Castro VD, Schroeder LF, Quirino BF et al (2013) Acidobacteria from oligotrophic soil from the Cerrado can grow in a wide range of carbon source concentrations. Can J Microbiol 59:746–753
Cayuela ML, Sánchezmonedero MA, Roig A et al (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions. Sci Rep 3:1732
Chen C, Phillips IR, Condron LM et al (2013) Impacts of greenwaste biochar on ammonia volatilisation from bauxite processing residue sand. Plant and Soil 367:301–312
Dai Z, **ong X, Zhu H et al (2021) Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3:239–254
Dangi S, Gao S, Duan Y et al (2020) Soil microbial community structure affected by biochar and fertilizer sources. Appl Soil Ecol 150:103452
Doydora SA, Cabrera et al (2011) Release of nitrogen and phosphorus from poultry litter amended with acidified biochar. Int J Environ Res Public Health 8:1494–1502
Duan PP, Zhang X, Zhang Q et al (2018) Field-aged biochar stimulated N2O production from greenhouse vegetable production soils by nitrification and denitrification. Sci Total Environ 642:1302–1310
Durenkamp M, Luo Y, Brookes PC (2010) Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biol Biochem 42:2026–2029
Eikmeyer FG, Kofinger P, Poschenel A (2013) Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J Biotechnol 167:334–343
Guan H, Yan Y, Li X (2018) Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour Technol 265:282–290
Harter J, Guzman-Bustamante I, Kuehfuss S et al (2016) Gas entrapment and microbial N2O reduction reduce N2O emissions from a biochar-amended sandy clay loam soil. Sci Rep 6:1–15
Kumuduni NP, James TFW, Yohey H et al (2019) Response of microbial communities to biochar-amended soils: A critical review. Biochar 1:3–22
Langille MG, Zaneveld J, Caporaso JG et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Li F, Liang X, He S et al (2020) Biochar slows gross nitrification and gasses N emission via lower autotrophic nitrification in paddy soils. J Soil Sediment 20:1–12
Lin S, Hernandez-Ramirez G (2021) Nitrogen turnover and N2O production in incubated soils after receiving field applications of liquid manure and nitrification inhibitors. Can J Soil Sci 101:1–15
Lin Y, Ding W, Liu D et al (2017) Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol Biochem 113:89–98
Liu X, Wang H, Liu C et al (2021) Biochar increases maize yield by promoting root growth in the rainfed region. Arch Agronom Soil Sci 67:1411–1424
Margesin F, Schinner (2005) Manual for Soil Analysis e Monitoring and Assessing Soil Bioremediation. J Chem Phys 55:20–27
Ni K, Wang F, Zhu B et al (2017) Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour Technol 238:706–715
Page AL, Robert H (1982) Methods of soil analysis. American Society of Agronomy
Shen JP, Zhang LM, Zhu YG et al (2008) Abundance and composition of ammonia-oxidizing bacteria and ammoniaoxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Sika MP, Hardie AG (2014) Effect of pine wood biochar on ammonium nitrate leaching and availability in a South African sandy soil. Eur J Soil Once 65:113–119
Singh BP, Hatton BJ, Singh B et al (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224–1235
Stanley EH, Rojas S, Lottig NR et al (2019) Comparison of total nitrogen data from direct and Kjeldahl-based approaches in integrated data sets. Limnol Oceanogr: Methods 17:639–649
Sun H, Zhang H, **ao H et al (2019) Wheat straw biochar application increases ammonia volatilization from an urban compacted soil giving a short-term reduction in fertilizer nitrogen use efficiency. J Soil Sediment 19:1624–1631
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang JY, Pan XJ, Liu Y et al (2012) Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant and Soil 360:287–298
Wang S, Wang ZF, Long Y, Gao M (2020) Effect of Fertilizer reduction and biochar application on soil nitrogen loss in purple upland. Environ Sci 41:2406–1415 (in chinese)
Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320:629–629
Xu HJ, Wang XH, Li H et al (2014) Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ Sci Technol 48:9391–9399
Yang L, Yuan X, Li J et al (2019) Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour Technol 275:280–287
Yang X, Lu Y, Ding Y et al (2017) Optimising nitrogen fertilisation: a key to improving nitrogen-use efficiency and minimising nitrate leaching losses in an intensive wheat/maize rotation. Field Crop Res 206:1–10
Yao Q, Liu JJ, Yu ZH et al (2017) Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl Soil Ecol 113:11–21
Zhang M, **a H, Lv B et al (2019) Short-term effect of biochar amendments on total bacteria and ammonia oxidizers communities in different type soils. Sci Agric Sinica 52:1260–1271 (in chinese)
Zhang YJ, Wu T, Zhao J et al (2017) Effect of biochar amendment on bacterial community structure and diversity in straw-amended soils. Acta Scientiae Circumstantiae 37:712–720 (in Chinese)
Zhou GX, Xu X, Qiu X et al (2019) Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour Technol 272:10–18
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the Henan Provincial Science and Technology Department Project (212102310076), Key research and development projects of Henan Province (221111520600) and Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2021JD17).
Author information
Authors and Affiliations
Contributions
All the authors collaborated for the completion of this work. Tian Hu designed and accomplished the first draft. Jun Zhang provided valuable insights and suggestions for this article. Jiating Wei, Li Du, and Jibao Chen were involved in the initial writing and editing of the manuscript. The authors have all read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All listed authors consented to the submission of this manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, T., Wei, J., Du, L. et al. The effect of biochar on nitrogen availability and bacterial community in farmland. Ann Microbiol 73, 4 (2023). https://doi.org/10.1186/s13213-022-01708-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-022-01708-1