Abstract
Background
Migratory birds can cross geographical and environmental barriers and are thereby able to facilitate transmission of tick-borne pathogens both as carriers of infected ticks and as reservoirs of pathogenic microorganisms. Ixodes ricinus is one of the most abundant tick species in the Northern Hemisphere and a main vector of several Babesia species, some which pose a potential threat to human and animal health. At present only two cases of overt babesiosis in humans have so far been reported in Sweden. To better understand the potential role of birds as disseminators of zoonotic Babesia protozoan parasites, we investigated the presence of Babesia species in ticks removed from migratory birds.
Methods
Ticks were collected from birds captured at Ottenby Bird Observatory, south-eastern Sweden, from March to November 2009. Ticks were molecularly identified to species, and morphologically to developmental stage, and the presence of Babesia protozoan parasites was determined by real-time PCR.
Results
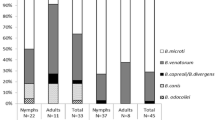
In total, 4601 migratory birds of 65 species were examined for tick infestation. Ticks removed from these birds have previously been investigated for the presence of Borrelia bacteria and the tick-borne encephalitis virus. In the present study, a total of 1102 ticks were available for molecular analysis of Babesia protozoan parasites. We found that 2.4% of the ticks examined, all I. ricinus, were positive for mammal-associated Babesia species. Out of all Babesia-positive samples, Babesia venatorum was the most prevalent (58%) species, followed by Babesia microti (38%) and Babesia capreoli (4.0%). B. venatorum and B. capreoli were detected in I. ricinus larvae, whereas B. microti was only present in I. ricinus nymphs. This supports the view that the two first-mentioned species are vertically (transovarially) transmitted in the tick population, in contrast to B. microti. The largest number of Babesia-infected ticks was removed from the common redstart (Phoenicurus phoenicurus) and European robin (Erithacus rubecula).
Conclusions
This study reveals that Babesia protozoan parasites are present in ticks infesting migratory birds in south-eastern Sweden, which could potentially lead to the dissemination of these tick-borne microorganisms into new areas, thus posing a threat to humans and other mammals.
Graphical Abstract

Similar content being viewed by others
Background
The role of migratory birds as hosts of disease vectors such as ticks and of potentially human pathogenic microorganisms has been increasingly recognized. Birds can cross geographical and environmental barriers and take part in the dispersal of bacteria, viruses, and protozoa [1]. Seasonal migration is especially pronounced in northern Europe, where a large part of the avifauna migrate, either to milder regions in western Europe or the Mediterranean region, or long distances to sub-Saharan Africa or even Asia. This means that birds returning in spring could carry ticks from regions that better sustain year-round transmission of tick-borne pathogens, and possibly contribute to reseeding foci of infections in temperate and boreal areas. Indeed, several studies in the Scandinavian countries have shown the presence of ticks and tick-borne infections in returning migratory birds, especially well-studied pathogens such as Borrelia burgdorferi sensu lato (s.l.) [2], Rickettsia spp. [3], and tick-borne encephalitis (TBE) virus [4]. However, the potential for less-studied, rarer, or unknown pathogens in bird-borne ticks to be detected in birds has come into focus recently, for instance with the bacterium Neoehrlichia mikurensis, which once identified has been shown to be fairly abundant in ticks [5]. Another pathogen of concern is Babesia spp., a protozoan parasite causing babesiosis—an emerging tick-borne human disease in the Holarctic region.
More than 100 species of Babesia have been described. The majority have been recorded in mammals, and 16 species have so far been described from avian hosts [6, 7]. In Europe, the roe deer (Capreolus capreolus) is considered the main vertebrate host of both Babesia capreoli and Babesia venatorum [8, 9], while Babesia divergens is prevalent in cattle in southern Sweden [10, 11]. Serological studies indicate that another species of Babesia—Babesia motasi—may be common in sheep herds in south-eastern Sweden [12]. Several of the mammal-associated species, such as B. divergens, Babesia duncani, B. venatorum, and Babesia microti, are zoonotic pathogens and of increasing medical importance, causing from asymptomatic infections to mild or serious, sometimes even fatal, human disease, particularly in immunocompromised persons [13,14,15,16,17,18]. In Europe, most of the severe cases of human babesiosis have involved infections of B. divergens [14, 15, 18,19,20]. However, the relatively recent discovery of the closely related B. venatorum [21, 22] may suggest that some of the earlier cases diagnosed as due to B. divergens may in fact have been caused by B. venatorum. During the last decades, the recorded incidence of human babesiosis due to B. microti, which is present in a reservoir of small mammals in the Holarctic region, has increased considerably in the north-eastern United States [23]. A few cases of human disease caused by B. microti or B. microti-like species have recently also been diagnosed in Europe and Asia [19, 24].
In nature, most mammalian Babesia spp. are transmitted by ixodid ticks [7, 25]. However, there is circumstantial evidence suggesting that some of the avian Babesia spp. may be vectored by soft ticks [7]. In North America, blood transfusion is recognized as an increasing and serious mode of accidental transmission of B. microti [26]. Human B. microti infection by blood transfusion has also been documented in Europe [24].
In Sweden, Ixodes ricinus is the most abundant tick species infesting humans [27], and also the most abundant tick species detected on passerine birds during migration in southern Sweden [2]. Three species of potentially zoonotic Babesia species—B. divergens, B. microti, and B. venatorum—have been recorded at prevalence rates of 0.2%, 3.2%, and 1.0%, respectively, in questing I. ricinus ticks in southern Sweden [28]. In a study that examined 2038 I. ricinus ticks which had been removed from humans in Sweden and on the Åland Islands, Finland, B. capreoli, B. microti, and B. venatorum were recorded in 0.25%, 1.60%, and 1.30% of the ticks, respectively [29].
The aim of this study is to better understand the potential role of migratory birds as disseminators of Babesia protozoan parasites and to determine the prevalence of Babesia spp. in ticks infesting birds during their spring and autumn migrations in south-eastern Sweden. Part of this study, related to data on the prevalence of Borrelia spp. and the TBE virus in ticks removed from migratory birds during 2009, has been reported elsewhere [30].
Methods
Sampling, analyses, and processing of ticks
Full details of the sampling site, procedure for bird captures and bird classification, determination of developmental stage and species of collected ticks, and total nucleic acid extraction from ticks and cDNA synthesis are available in the previous report [30].
In short, ticks were collected from birds captured during the periods 15 March–15 June and 15 July–15 November 2009 at the Ottenby Bird Observatory, which is located on the southern point of the island of Öland in south-eastern Sweden (56° 12′ N, 16° 24′ E). Trapped birds were identified to species level and classified into residents, short-distance migrants, partial migrants, and long-distance migrants. Any collected tick was photographed and morphologically identified to stage of development (larva, nymph, or adult), and sex of adults. Each tick was individually homogenized using a TissueLyser II (Qiagen) followed by extraction, purification, and isolation of total nucleic acids using MagAttract® Viral RNA M48 kit in a BioRobot M48 workstation (Qiagen). The total nucleic acids were reverse-transcribed to cDNA using the illustra™ Ready-to-Go RT-PCR Beads kit (GE Healthcare, Amersham Place, UK), which served as template in all the polymerase chain reaction (PCR) assays. To identify the genus and species of the ticks, each specimen was analysed by a PCR method targeting the tick mitochondrial 16S rRNA gene followed by DNA sequencing, as previously described [30].
Sampling of birds was approved by the Swedish Board of Agriculture, delegated through the Animal Research Ethics Committee in Linkö** (decision 43–09).
Detection and determination of Babesia species
Detection of Babesia spp. was done using a SYBR green real-time PCR assay, as previously described [10]. Primers BJ1 (5′–GTC TTG TAA TTG GAA TGA TGG–3′) and BN2 (5′–TAG TTT ATG GTT AGG ACT ACG–3′) were designed to target the Babesia 18S rRNA gene to amplify a 411–452-bp long amplicon depending on the species of Babesia [31].
A 20-μl reaction consisted of 10 µl SYBR™ Green PCR Master Mix (Thermo Fisher Scientific, Stockholm, Sweden), 0.4 µl of each primer (10 µM; Invitrogen), 6.2 µl RNase-free water, and 3 µl cDNA template. The PCR template of 3 µl consisted of a pool of cDNA from three tick specimens per reaction. A positive PCR control, consisting of 3 µl B. microti DNA (10 ng/µl) extracted from an I. ricinus tick collected in Slovakia, was included in each run. The B. microti DNA was kindly provided by Dr Bronislava Víchová (Institute of Parasitology, Slovak Academy of Sciences, Slovakia) through Dr Martin Andersson (Centre for Ecology and Evolution in Microbial Model Systems, Linnaeus University, Kalmar, Sweden). As a negative control in the PCR assay, RNase-free water was used as template. When Babesia-positive pools were detected, the samples were re-analysed individually.
The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using an activation step at 94 °C for 10 min, and 35 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min, and finally one cycle of 72 °C for 5 min. Immediately after completion of PCR, melting curve analyses were performed by heating to 95 °C for 15 s, followed by cooling to 60 °C for 1 min, and subsequent heating to 95 °C at 0.8 °C min−1 with continuous fluorescence recording.
To determine the species of Babesia in the PCR-positive samples, nucleotide sequencing of the PCR-products was performed by Macrogen Inc. (Amsterdam, The Netherlands). All sequences obtained were confirmed by sequencing both strands. The obtained chromatograms were initially edited and analysed using BioEdit Software v7.0 (Tom Hall, Ibis Therapeutics, Carlsbad, CA, USA), and the sequences were examined using the Basic Local Alignment Search Tool (BLAST). Sequences obtained have been deposited in GenBank with accession numbers ranging from MW554592 to MW554617.
Species determination of B. microti and B. venatorum is possible by sequencing the amplicon from the real-time PCR assay. B. capreoli is highly similar to B. divergens, and the two species differ only at three nucleotide positions at the 18S rRNA gene, specifically on positions 631, 663, and 1637 (99.83% nucleotide similarity) [32]. The two first positions are included in the DNA fragment amplified by the primers used in this study. Additional file 1 shows all the aligned Babesia nucleotide sequences.
Statistical analyses
Data were presented as percentages for categorical variables. The categorical variables were analysed using the chi-square test, but when the expected frequency was < 5 in at least one of the cells of the contingency table, Fisher’s exact test was used. Statistical analyses were performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA). P values ≤ 0.05 were considered statistically significant.
Results
Ticks collected from birds and ticks available for PCR analyses
A total of 4601 bird individuals (4788 bird captures) of 65 species were examined for ticks at least once during the study period at the Ottenby Bird Observatory. A total of 749 bird individuals (759 bird captures) of 35 species were infested with a total of 1339 ticks (Table 1). These results were previously reported in the study of Wilhelmsson et al. [30].
In this study, 1102 ticks (i.e., cDNA samples) were available for analysis. Of these, 543 were larvae, 552 nymphs and two ticks were adult females. Five ticks could not be determined to developmental stage due to lack of photos. The ticks available for analysis were molecularly identified to: I. ricinus (n = 1,051; 514 larvae, 532 nymphs, 5 specimens of unknown developmental stage), I. frontalis (n = 24; 8 larvae, 15 nymphs, 1 adult female), Haemaphysalis punctata (n = 12; 12 larvae), and Hyalomma marginatum (n = 4; 1 larva, 2 nymphs, 1 adult female). The remaining 11 ticks could not be molecularly identified to species due to unreadable sequences despite several sequencing attempts. Instead, these 11 ticks were only determined to genus level, Ixodes (8 larvae, 3 nymphs), based on the photos.
Prevalence of Babesia species in ticks removed from birds
Of the ticks analysed, 2.4% (26/1,102) were positive for Babesia spp. All Babesia-positive ticks belonged to I. ricinus (26/1,051). Of these, 2.0% of the I. ricinus larvae were Babesia-positive in the PCR-assay (10/514) and 3.0% of the I. ricinus nymphs were Babesia-positive (16/532). All five I. ricinus ticks that could not be determined to developmental stage were Babesia-negative. No significant difference in Babesia prevalence between larvae and nymphs was detected.
Three species of Babesia were identified: B. venatorum (n = 15), B. microti (n = 10), and B. capreoli (n = 1). B. venatorum was detected in 9 larvae and 6 nymphs, B. microti in 10 nymphs, and B. capreoli in 1 larva (Table 2).
Of all samples that were determined to Babesia species level (n = 26; Table 2), 17 were detected in ticks captured in spring (15 March–15 June), and 9 were detected in ticks in late summer–autumn (15 July–15 November). No significant difference was detected between the proportions of Babesia-positive ticks collected in spring (17/495) and Babesia-positive ticks collected in late summer–autumn (9/556).
Bird species with ticks positive for Babesia species
Babesia-positive I. ricinus ticks were removed from eight bird species in spring or in late summer–autumn (Table 3): the common redstart (Phoenicurus phoenicurus, n = 7), European robin (Erithacus rubecula, n = 7), common blackbird (Turdus merula, n = 5), Eurasian wren (Troglodytes troglodytes, n = 2), tree pipit (Anthus trivialis, n = 2), common starling (Sturnus vulgaris, n = 1), common whitethroat (Sylvia communis, n = 1), and lesser whitethroat (Sylvia curruca, n = 1).
Discussion
So far, only a few studies have investigated tick-borne pathogens in ticks, which are blood-feeding on migratory birds in northern Europe, and the current study adds new information to this subject. Our study is the first one to report the presence of Babesia species in ticks collected from birds in Sweden.
The prevalence of Babesia spp. detected in this study (2.4%) is on a similar level as what has been reported in ticks collected from birds in other countries in northern Europe, i.e., Norway, northern Germany, and north-western Russia (1.0–4.7%) [33,34,35]. In those studies, the Babesia species were identified as B. venatorum, B. microti, and B. divergens. In our study, B. venatorum was the most prevalent species and was detected in nymphs as well as in larvae of I. ricinus. Both B. venatorum [8] and B. divergens [36] are known to be transovarially transmitted. Thus, their presence in tick larvae should not be taken as an indication that the pathogen was derived from feeding upon the avian hosts. To elucidate that, one would need to investigate the presence of Babesia spp. in blood taken directly from the birds. Our data suggest that B. capreoli is also transovarially transmitted in I. ricinus ticks, since it was present in a larval tick. This was expected, given the close phylogenetic relationship between B. divergens and B. capreoli and since it is a trait regarded as common to all members of the genus Babesia sensu stricto [19].
B. microti, on the other hand, is not considered to be vertically transmitted [19], and was only found in nymphs. In contrast to our results, Franke et al. [37] and Hildebrandt et al. [34] recorded B. microti in I. ricinus larvae removed from several passerine bird species in Germany. We are not aware of any other reports where tick larvae removed from birds have been shown to harbour B. microti. On the contrary, the different taxa included in the B. microti complex are generally considered to have different species of small mammals, specifically rodents and shrews, as their main vertebrate reservoirs [13, 19, 38,39,40,41,42,43]. It might be possible that the B. microti infections recorded by Franke et al. [37] and Hildebrandt et al. [34] in larvae of I. ricinus were acquired by co-feeding transmission from nymphal or adult ticks that had previously fed on B. microti-infected mammals. However, experimental evidence from North America supports the notion that birds can act as reservoirs of B. microti: Hersh and colleagues tested 10 North American mammal species and four bird species for reservoir competency, i.e., capacity to infect the main vector in North America, Ixodes scapularis, with B. microti. They found reservoir competence levels of > 17% in white-footed mouse (Peromyscus leucopus), racoon (Procyon lotor), short-tailed shrew (Blarina brevicauda), and eastern chipmunk (Tamias striatus), and < 6% but > 0% in all other species, including all four bird species tested including three species belonging to the Turdidae family [44]. There is significant genetic heterogeneity among strains of B. microti [41,42,43, 45]. Therefore, further genetic investigations are necessary as well as studies to explore whether bird species even in Europe are systemically infected, competent reservoirs (transmission hosts) for any of these taxa of “B. microti”.
The tick species H. punctata and Hy. marginatum have been reported to harbour different Babesia species, where B. motasi, B. bovis, B. bigemina, B. caballi and B. major have been detected in H. punctata, and B. microti has been detected in Hy. marginatum [46,47,48,49]. A serological survey of sheep indicated that B. motasi may be present on the island of Gotland, south-eastern Sweden, where it is presumably vectored by H. punctata [12]. To our knowledge, neither H. punctata nor Hy. marginatum ticks removed from birds have been investigated previously for the presence of Babesia species. However, in the present study, only I. ricinus ticks were positive for Babesia. This is not surprising, given the overall low prevalence of Babesia spp. detected in the I. ricinus ticks, and the small number of specimens of the other tick species analysed.
In this study, the Babesia-positive ticks were found on eight common passerine bird species. Given the location, all these individuals are considered to be on active migration. Four of the species—European robin (Erithacus rubecula), common starling (Sturnus vulgaris), common blackbird (Turdus merula), and Eurasian wren (Troglodytes troglodytes)—are common and widespread short-distance migrant (or partial migrant) species that occur in a range of habitats, including in the vicinity of humans, in gardens, forests, and pastures. The other four species are obligatory long-distance migrants wintering in sub-Saharan Africa, predominantly West Africa, and include tree pipit (Anthus trivialis), common redstart (Phoenicurus phoenicurus), common whitethroat (Sylvia communis), and lesser whitethroat (Sylvia curruca), and although common, they are less associated with human settlements [50]. Interestingly, 17 of the bird individuals infested by Babesia-positive ticks were captured during their northward spring migration, suggesting a potential role of these birds as disseminators of Babesia protozoan parasites into Sweden. Although the prevalence of Babesia spp. was low, the sheer number of birds involved in migration would imply a significant number of introductions of infected ticks on an annual basis. Quantifying this number would require larger sample sizes and a wider sampling of host species. However, the presence of infected ticks in common passerine species that occur in habitats also frequented by humans and domesticated animals suggests that the risk of encountering potentially Babesia-infective ticks exists. However, the risk to humans resulting from these introductions remains speculative, especially in relation to the enzootic occurrence of these pathogens.
Two of the three Babesia species, i.e., B. venatorum and B. microti, detected in this study are previously known to cause human disease. In Europe, the most common cause of clinical human babesiosis is B. divergens, which typically is diagnosed in immunocompromised individuals and often gives rise to a severe illness [16, 51]. Human disease caused by B. divergens has also been reported in immunocompetent patients [52]. A few cases of B. microti and B. venatorum infection have also been reported in Europe [21, 22, 24, 53]. In contrast to B. divergens, B. venatorum, and B. microti, B. capreoli is considered not to be human-pathogenic [32].
In southern Sweden, a prevalence of 2.5% for B. microti and/or B. divergens antibodies among healthy individuals was recorded, and an even higher seroprevalence (16.3%) among seropositive Borrelia burgdorferi sensu lato patients was detected [54]. Despite the high seroprevalence, only two cases of human babesiosis have so far been reported in Sweden [18, 53]. Thus, the risk of develo** severe babesiosis after an I. ricinus tick bite among healthy individuals in Sweden appears to be low [29]. However, as pointed out previously by others [19, 25, 54], we cannot exclude that this potentially severe infection is underdiagnosed.
Conclusions
This is the first study showing the presence of B. venatorum, B. microti, and B. capreoli in ticks removed from birds in Sweden. The study also reveals that zoonotic Babesia species are present in ticks infesting migratory birds in south-eastern Sweden, which could lead to dissemination of these tick-borne microorganisms into new areas.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Raw data can be shared with researchers upon a specific request.
References
Hasle G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front Cell Infect Microbiol. 2013;3:48.
Olsen B, Jaenson TG, Bergstrom S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;61(8):3082–7.
Elfving K, Olsen B, Bergstrom S, Waldenstrom J, Lundkvist A, Sjostedt A, et al. Dissemination of spotted fever rickettsia agents in Europe by migrating birds. PLoS ONE. 2010;5(1):e8572.
Waldenstrom J, Lundkvist A, Falk KI, Garpmo U, Bergstrom S, Lindegren G, et al. Migrating birds and tickborne encephalitis virus. Emerg Infect Dis. 2007;13(8):1215–8.
Labbe Sandelin L, Tolf C, Larsson S, Wilhelmsson P, Salaneck E, Jaenson TG, et al. Candidatus Neoehrlichia mikurensis in ticks from migrating birds in Sweden. PLoS ONE. 2015;10(7):e0133250.
Yabsley MJ, Vanstreels RET, Shock BC, Purdee M, Horne EC, Peirce MA, et al. Molecular characterization of Babesia peircei and Babesia ugwidiensis provides insight into the evolution and host specificity of avian piroplasmids. Int J Parasitol Parasites Wildl. 2017;6(3):257–64.
Peirce MA. A taxonomic review of avian piroplasms of the genus Babesia Starcovici, 1893 (Apicomplexa: Piroplasmorida: Babesiidae). J Nat Hist. 2000;34(3):317–32.
Bonnet S, Jouglin M, L’Hostis M, Chauvin A. Babesia sp EU1 from roe deer and transmission within Ixodes ricinus. Emerg Infect Dis. 2007;13 (8):1208–10.
Andersson MO, Bergvall UA, Chirico J, Christensson M, Lindgren PE, Nordstrom J, et al. Molecular detection of Babesia capreoli and Babesia venatorum in wild Swedish roe deer Capreolus capreolus. Parasit Vectors. 2016;9:221.
Andersson MO, Víchová B, Tolf C, Krzyzanowska S, Waldenstrom J, Karlsson ME. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos taurus). Sweden Ticks Tick Borne Dis. 2017;8(6):933–5.
Christensson DA. Clinical and serological response after experimental inoculation with Babesia divergens of newborn calves with and without maternal antibodies. Acta Vet Scand. 1987;28(3–4):381–92.
Christensson D, Thunegard E. Babesia motasi in sheep on the island of Gotland in Sweden. Vet Parasitol. 1981;9(2):99–106.
Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13(3):451–69.
Morch K, Holmaas G, Frolander PS, Kristoffersen EK. Severe human Babesia divergens infection in Norway. Int J Infect Dis. 2015;33:37–8.
Haapasalo K, Suomalainen P, Sukura A, Siikamaki H, Jokiranta TS. Fatal babesiosis in man, Finland, 2004. Emerg Infect Dis. 2010;16(7):1116–8.
Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29(2):357–70.
Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30(12–13):1323–37.
Uhnoo I, Cars O, Christensson D, Nystrom-Rosander C. First documented case of human babesiosis in Sweden. Scand J Infect Dis. 1992;24(4):541–7.
Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1(1):3–10.
Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16(4):622–36.
Herwaldt BL, Caccio S, Gherlinzoni F, Aspock H, Slemenda SB, Piccaluga P, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9(8):942–8.
Haselbarth K, Tenter AM, Brade V, Krieger G, Hunfeld KP. First case of human babesiosis in Germany-Clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol. 2007;297(3):197–204.
CDC: Centers for Disease Control and Prevention. Parasites Babesiosis. Data & Statistics. Number and incidence of reported cases of babesiosis, by state and year, 2011–2018. https://www.cdc.gov/parasites/babesiosis/data-statistics/index.html (2020). Accessed 19 Aug 2020.
Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26(8):595–601.
Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38(11):1219–37.
Fang DC, McCullough J. Transfusion-transmitted Babesia microti. Transfus Med Rev. 2016;30(3):132–8.
Wilhelmsson P, Lindblom P, Fryland L, Nyman D, Jaenson TG, Forsberg P, et al. Ixodes ricinus ticks removed from humans in Northern Europe: seasonal pattern of infestation, attachment sites and duration of feeding. Parasit Vectors. 2013;6(1):362.
Karlsson ME, Andersson MO. Babesia species in questing Ixodes ricinus. Sweden Ticks Tick Borne Dis. 2016;7(1):10–2.
Wilhelmsson P, Lovmar M, Krogfelt KA, Nielsen HV, Forsberg P, Lindgren PE. Clinical/serological outcome in humans bitten by Babesia species positive Ixodes ricinus ticks in Sweden and on the Åland Islands. Ticks Tick Borne Dis. 2020:101455.
Wilhelmsson P, Jaenson TGT, Olsen B, Waldenstrom J, Lindgren PE. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: a seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasit Vectors. 2020;13(1):607.
Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13(1):65–70.
Malandrin L, Jouglin M, Sun Y, Brisseau N, Chauvin A. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. 2010;40(3):277–84.
Hasle G, Leinaas HP, Roed KH, Oines O. Transport of Babesia venatorum-infected Ixodes ricinus to Norway by northward migrating passerine birds. Acta Vet Scand. 2011;53:41.
Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E. The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 2010;1(2):105–7.
Movila A, Reye AL, Dubinina HV, Tolstenkov OO, Toderas I, Hubschen JM, et al. Detection of Babesia Sp. EU1 and members of spotted fever group rickettsiae in ticks collected from migratory birds at Curonian Spit North-Western Russia. Vector Borne Zoonotic Dis. 2011;11(1):89–91.
Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, L’Hostis M, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134(Pt 2):197–207.
Franke J, Fritzsch J, Tomaso H, Straube E, Dorn W, Hildebrandt A. Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in Central Germany. Appl Environ Microbiol. 2010;76(20):6829–36.
Welc-Falęciak R, Bajer A, Behnke JM, Siński E. Effects of host diversity and the community composition of hard ticks (Ixodidae) on Babesia microti infection. Int J Med Microbiol. 2008;298:235–42.
Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents–analyzing the host-pathogen-vector interface in a metropolitan area. Parasit Vectors. 2012;5:191.
Young KM, Corrin T, Wilhelm B, Uhland C, Greig J, Mascarenhas M, et al. Zoonotic Babesia: A sco** review of the global evidence. PLoS ONE. 2019;14(12):e0226781.
Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2013;2:18–31.
Goethert HK, Telford SR 3rd. What is Babesia microti? Parasitology. 2003;127(Pt 4):301–9.
Telford SR 3rd, Goethert HK. Emerging tick-borne infections: rediscovered and better characterized, or truly ’new’ ? Parasitology. 2004;129(Suppl):S301–27.
Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18(12):1951–7.
Gray JS. Identity of the causal agents of human babesiosis in Europe. Int J Med Microbiol. 2006;296(Suppl 40):131–6.
García-Sanmartín J, Barandika JF, Juste RA, García-Pérez AL, Hurtado A. Distribution and molecular detection of Theileria and Babesia in questing ticks from northern Spain. Med Vet Entomol. 2008;22(4):318–25.
Uilenberg G. Babesia–a historical overview. Vet Parasitol. 2006;138(1–2):3–10.
Nowak-Chmura M, Siuda K. Ticks of Poland. Review of contemporary issues and latest research. Ann Parasitol. 2012;58(3):125–55.
Morzaria SP, Bland P, Brocklesby DW. Ultrastructure of Babesia major in the tick Haemaphysalis punctata. Res Vet Sci. 1976;21(1):1–11.
Busse P. European passerine migration system – what is known and what is lacking. Ring. 2001;23:3–36.
Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366(25):2397–407.
Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, et al. Babesiosis in immunocompetent patients. Europe Emerg Infect Dis. 2011;17(1):114–6.
Blackberg J, Lazarevic VL, Hunfeld KP, Persson KEM. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann Hematol. 2018;97(4):731–3.
Svensson J, Hunfeld KP, Persson KEM. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick Borne Dis. 2019;10(1):186–90.
Acknowledgements
We would like to thank the staff members at the Ottenby Bird Observatory for species identification of the birds and for collecting the ticks attached to the birds. We are also grateful to Matilda Karlsson for skilful laboratory assistance. The first author OP would like to thank Professor Krzysztof Solarz from the Department of Parasitology, Medical University of Silesia in Katowice, Poland, for his support. This is contribution no. 319 from Ottenby Bird Observatory.
Funding
Open access funding provided by Linkö** University.. This research was supported as part of NorthTick, an Interreg project supported by the North Sea Programme of the European Regional Development Fund of the European Union. This study was also supported by the EU Interreg ÖKS V program ScandTick Innovation, project ID 20200422, reference no. 2015–000167, and by The Swedish Research Council Branch of Medicine (grant no. K2008-58X-14631–06-3), Carl Tryggers stiftelse, Helge Ax:son Johnsons stiftelse, Längmanska Kulturfonden, Magnus Bergvalls stiftelse, the Medical Research Council of Southeast Sweden (FORSS-657881, FORSS-931010), and by the Division of Laboratory Medicine, Region Jönkö** County. This work was carried out under the auspices of ESGBOR (the European Study Group on Lyme Borrelioses) and VectorNet, a European network for sharing data on the geographic distribution of arthropod vectors, transmitting human and animal disease agents (framework contract OC/EFSA/AHAW/2013/02-FWC1) funded by the European Food Safety Authority (EFSA) and the European Centre for Disease prevention and Control (ECDC).
Author information
Authors and Affiliations
Contributions
PL, PF, BO, and JW planned the study and organized the collection of ticks at the Ottenby Bird Observatory. TGTJ determined the developmental stage of each tick and sex of the adult ticks. OP and PW performed the laboratory analyses, processed the data, and wrote the manuscript together with TGTJ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Sampling of birds was approved by the Swedish Board of Agriculture, delegated through Animal Research Ethics Committee in Linkö** (decision 43–09). Ethical approval is not required for this study, because the analyses were performed on cDNA from ticks, which were removed from birds in accordance with the animal welfare guidelines and regulations.
Consent for publication
All authors have given consent for publication of this manuscript in the Parasites & Vectors journal.
Competing interests
PL has been an external scientific expert to Valneva Austria GmbH, Vienna, Austria. All other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
The aligned Babesia nucleotide sequences based on PCR-products.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wilhelmsson, P., Pawełczyk, O., Jaenson, T.G.T. et al. Three Babesia species in Ixodes ricinus ticks from migratory birds in Sweden. Parasites Vectors 14, 183 (2021). https://doi.org/10.1186/s13071-021-04684-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04684-8