Abstract
Background
Extubation failure is associated with increased mortality. Cough ineffectiveness may be associated with extubation failure, but its quantification for patients undergoing weaning from invasive mechanical ventilation (IMV) remains challenging.
Methods
Patients under IMV for more than 24 h completing a successful spontaneous T-tube breathing trial (SBT) were included. At the end of the SBT, we performed quantitative sonometric assessment of three successive coughing efforts using a sonometer. The mean of the 3-cough volume in decibels was named Sonoscore.
Results
During a 1-year period, 106 patients were included. Median age was 65 [51–75] years, mainly men (60%). Main reasons for IMV were acute respiratory failure (43%), coma (25%) and shock (17%). Median duration of IMV at enrollment was 4 [3–7] days. Extubation failure occurred in 15 (14%) patients. Baseline characteristics were similar between success and failure extubation groups, except percentage of simple weaning which was lower and MV duration which was longer in extubation failure patients. Sonoscore was significantly lower in patients who failed extubation (58 [52–64] vs. 75 [70–78] dB, P < 0.001). After adjustment on MV duration and comorbidities, Sonoscore remained associated with extubation failure. Sonoscore was predictive of extubation failure with an area under the ROC curve of 0.91 (IC95% [0.83–0.99], P < 0.001). A threshold of Sonoscore < 67.1 dB predicted extubation failure with a sensitivity of 0.93 IC95% [0.70–0.99] and a specificity of 0.82 IC95% [0.73–0.90].
Conclusion
Sonometric assessment of cough strength might be helpful to identify patients at risk of extubation failure in patients undergoing IMV.
Similar content being viewed by others
Introduction
Invasive mechanical ventilation (IMV) is a common support organ therapy in intensive care unit (ICU). Given that prolonged IMV is associated with increased mortality, its duration has to be as limited as possible to reduce exposure to IMV-related complications [1, 2]. Nevertheless, extubation failure (EF) is also associated with a significant increase in mortality. It is therefore of paramount importance to develop easy-to-use tools at the bedside to evaluate the risk of EF [3].
Several trials suggested that ineffective cough is associated with EF in patients undergoing IMV owing to impaired airway clearance leading to secretion accumulation, atelectasis and secondary respiratory tract infection [4, 5]. An International Consensus Conference published in 2007 [6] highlighted that appropriate cough is required for extubation but the optimal way to assess cough efficiency is still debated [7, 8].
In this study, we hypothesized that quantitative sonometric assessment of the cough in patients who successfully passed a spontaneous breathing trial (SBT) might help to identify those at high risk of EF.
Methods
Population and weaning protocol
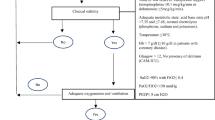
We conducted a prospective observational study in an 18-bed medical ICU in France (Saint-Antoine Hospital, Assistance Publique–Hôpitaux de Paris) from October 2021 to October 2022. Every adult patient requiring IMV for more than 24 h was eligible. According to local practice, standard oxygen therapy was the only oxygen delivery strategy applied after extubation. Exclusion criteria were limited to patients unable to cough 3 times to order, patients exhibiting an upper airway obstruction or requiring a reintubation for surgery or endoscopy. Only the extubation following the first successful SBT was solely analyzed. Ready-to-wean criteria and SBT failure criteria relied on guidelines from the International Consensus Conference on Weaning [6]. Prophylactic non-invasive ventilation (NIV) was standardized and used if patients were at high risk of EF as proposed by Rochwerg et al. [9].
The primary outcome was extubation failure, defined as reintubation within 48 h after extubation or as initiation of unplanned NIV or curative NIV in patients already receiving prophylactic NIV within the 48 h after extubation. Indication for reintubation, unplanned or curative NIV was post-extubation acute respiratory failure, defined by the presence of one or more of the following criteria, persistent over 5 min: SpO2 < 90% with an oxygen support ≥ 5 L/min, a respiratory rate ≥ 35/min, a pH < 7.35 with a pCO2 > 45 mmHg and/or use of accessory respiratory muscles. High flow nasal oxygen (HFNO) use was let at the clinician’s discretion.
Cough assessment
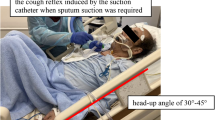
At the end of successful SBT, once considered ready for extubation by the attending physician, measurement was performed after endotracheal aspiration just before extubation. Cough strength, estimated by its sound level (decibels), was assessed by Pulsar Model 14® Sound-Level Meter (Additional file 1: Fig. S1) placed 2 cm away from the distal part of the endotracheal tube. Patients were asked to cough three times in a row in a maximum effort, allowing estimating the Sonoscore (i.e., mean in decibel produced by the three consecutive coughing efforts). Simultaneously, cough effectiveness was subjectively evaluated by the caregivers including nurse, resident and senior physician in charge of the patient using a three-levels scale (0, ineffective cough; 1, moderate cough; 2, effective cough). All caregivers were blinded to Sonoscore throughout the study.
Statistical analysis
Continuous variables were expressed as median [IQR], and categorical variables were expressed as absolute and relative frequencies. To assess associations between patient characteristics and EF, we first performed univariate analyses based on the two-tailed Mann–Whitney test or chi-squared test as appropriate. To identify independent predictors of EF, variables with P values less than 0.05 by univariate analysis and known factors associated with EF were included in a multivariable logistic regression model. The Kolmogorov–Smirnov test was used to evaluate the normality distribution of the continuous variables. The model's goodness of fit was assessed using the Hosmer–Lemeshow test and the discrimination by the area under the receiver operating characteristic curve (ROC-AUC). We used Youden’s method to determine the best cut-off predictive value of Sonoscore for EF. All tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Study population
From October 2021 to October 2022, 114 patients met the inclusion criteria. Of these, 8 patients were excluded from the analysis (Additional file 1: Fig. S2) and 106 patients were finally included, mainly male (60%), with a median age of 65 [51–75] years. The main characteristics at admission are depicted in Table 1.
Extubation outcome
Extubation failure occurred in 15 (14%) patients, 8 were reintubated and 7 required NIV (4 patients with unplanned NIV and 3 patients with curative from prophylactic NIV, with a median duration of 96 [58–114] hours). Baseline clinical and biological characteristics were similar between extubation success and failure groups, except for 2 parameters: the ratio of simple weaning which were lower and the duration of IMV longer in failure patients (Table 1).
Sonometric assessment of cough
Sonoscore was significantly lower in patients who failed extubation at H48 (with 58 [52–64] vs.75 [70–78] dB, median difference - 17 [- 19–- 11] dB, P < 0.001). Similar results were observed when extubation outcome was evaluated at day 7 (Fig. 1). Dynamic of cough was of interest because cough-related sound level significantly increased between the first and the third coughing effort (median level 73 [66–78] vs.76 [71–80] dB, median difference + 3 [1–5] dB, P < 0.001) in the success group whereas sound level did not change and remained low in patients who failed extubation (median level 59 [52–63] vs.60 [54–64] dB, median difference + 1 [0–4] dB, P = 0.187) (Additional file 1: Fig. S3). The Sonoscore did not change according to the duration of IMV.
Sonoscore was an accurate tool to predict EF with an AUC at 0.91 IC95% [0.83–0.99] P < 0.001), significantly higher (P = 0.014, Delong’s test) than the caregiver’s subjective assessment of cough (AUC 0.84 IC95% [0.73–0.94] P < 0.001) (Fig. 2). A threshold of Sonoscore at 67.1dB predicted EF with a sensitivity of 0.93 IC95% [0.70–0.99] and a specificity of 0.82 IC95% [0.73–0.90]. In a multivariate logistic regression model including mechanical ventilation duration in days and presence of cardiac and/or lung disease, Sonoscore remained independently associated with extubation failure (OR = 51.9 [9.05–988], P < 0.001) (Additional file 1: Fig. S4).
Discussion
In a monocentric prospective cohort including ICU patients under IMV, we found that quantitative assessment of cough using sonometry was a promising tool to identify patients at high risk of extubation failure.
Weaning from mechanical ventilation is a crucial step in the management of ICU patients, representing up to 50% of total duration of IMV [10, 11]. Decision to extubate is of paramount importance because post-extubation acute respiratory failure requiring mechanical ventilation is associated with significantly higher mortality [12]. Previous works have shown that EF is related to a respiratory cause in more than 50% of cases, ineffective cough and weakness of respiratory muscles being reported as the main drivers for reintubation [13]. Effective cough as important criteria for extubation has been proposed by experts [6], but its quantitative assessment remains difficult. Cough has been evaluated using a peak flow meter (i.e.,pneumotachograph or electronic flowmeter) but its predictive value for EF is not optimal (sensitivity of 79% and specificity of 71%) [14, 15]. In our work, we used a sonometer device allowing accurate quantification of the sound generated during cough effort assuming that the sonometric assessment of the cough is a surrogate of its strength. Here, we showed that a standardized sonometric evaluation of the cough at the bedside and more specifically the Sonoscore may provide additional insight from caregivers’ assessment to identify more accurately patients at risk of EF. Weaning process should aim to discriminate whether patients are ready to be extubated or not. In order to avoid the excess mortality associated with EF, we chose a Sonoscore threshold at 67.1 dB to predict EF with the best sensitivity/specificity combination as well as the highest sensitivity.
The duration of IMV positively correlates with respiratory muscle weakness [16, 17]. However, in our study, we did not find any association between the Sonoscore and the duration of MV. This suggests the role of a pre-existing inability to ensure a strong cough in EF patients, which would not be entirely explained by ICU-acquired weakness alone. Most studies focus on inspiratory muscle dysfunction during weaning [18,19,20], while abdominal wall muscles play an important role in active expiration and cough effectiveness. In contrast, the impact of IMV on expiratory muscles function has been less investigated and interventions targeting these muscles are scarce.
Our study has several limitations. First, the sample size is limited and results have to be confirmed in a larger cohort. Second, sonometric assessment of cough has been performed on a T-piece SBT, but its predictive value in patients with pressure-support ventilation SBT needs to be evaluated. Third, although no difference was observed regarding the abundance of secretions between patients with extubation success or failure, whether sonometric assessment of cough is affected in hypersecretive patients remains unclear. Fourth, cough assessment requires the patient’s cooperation. Fifth, despite its good prognostic value, Sonoscore does not provide pathophysiological information on the mechanism leading to extubation failure. Finally, in our study, we did not measure cough peak flow. Comparing the predictive value of Cough Peak Flow versus sonometric assessment should be evaluated in a future work.
Conclusion
In a monocentric prospective cohort including non-selected ICU patients under IMV who succeeded SBT, we found that objective cough assessment using sonometry may help to identify patients at high risk of extubation failure. Those results must be validated in a larger cohort.
Availability of data and materials
The datasets used and/or analyzed as well GraphPad sheets used in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- IMV:
-
Invasive mechanical ventilation
- ICU:
-
Intensive care unit
- EF:
-
Extubation failure
- SBT:
-
Spontaneous breathing trial
- NIV:
-
Non-invasive ventilation
- dB:
-
Decibel
- IQR:
-
Inter-quartile range
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- SAPS:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment
- PEEP:
-
Positive end-expiratory pressure
- FiO2:
-
Fraction of inspired oxygen
- PaCO2:
-
Partial pressure of arterial carbon dioxide
References
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–13.
De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med sept. 2007;35(9):2007–15.
Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–8.
Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30(7):1334–9.
Gonçalves MR, Honrado T, Winck J, Paiva J. Effects of mechanical insufflation-exsufflation in preventing respiratory failure after extubation: a randomized controlled trial. Crit Care. 2012;16(2):R48.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–56.
Duan J, Zhang X, Song J. Predictive power of extubation failure diagnosed by cough strength: a systematic review and meta-analysis. Crit Care. 2021;25(1):357.
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–70.
Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J août. 2017;50(2):1602426.
Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–9.
Esteban A, Alia I, Ibañez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. Chest. 1994;106(4):1188–93.
Thille AW, Richard JCM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–302.
Thille AW, Gacouin A, Coudroy R, Ehrmann S, Quenot JP, Nay MA, et al. Spontaneous-breathing trials with pressure-support ventilation or a T-piece. N Engl J Med. 2022;387(20):1843–54.
Almeida CM, Lopes AJ, Guimarães FS. Cough peak flow to predict the extubation outcome: comparison between three cough stimulation methods. Can J Respir Ther. 2020;20(56):58–64.
Beuret P, Roux C, Auclair A, Nourdine K, Kaaki M, Carton MJ. Interest of an objective evaluation of cough during weaning from mechanical ventilation. Intensive Care Med juin. 2009;35(6):1090–3.
Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact—a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–9.
Saccheri C, Morawiec E, Delemazure J, Mayaux J, Dubé BP, Similowski T, et al. ICU-acquired weakness, diaphragm dysfunction and long-term outcomes of critically ill patients. Ann Intensive Care. 2020;10(1):1.
Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome. Chest. 2017;152(6):1140–50.
Dres M, Rozenberg E, Morawiec E, Mayaux J, Delemazure J, Similowski T, et al. Diaphragm dysfunction, lung aeration loss and weaning-induced pulmonary oedema in difficult-to-wean patients. Ann Intensive Care. 2021;11(1):99.
Dres M, De Abreu MG, Merdji H, Müller-Redetzky H, Dellweg D, Randerath WJ, et al. Randomized clinical study of temporary transvenous phrenic nerve stimulation in difficult-to-wean patients. Am J Respir Crit Care Med. 2022;205(10):1169–78.
Acknowledgements
The authors want to thank the Saint-Antoine’s Hospital medical intensive care team for her precious support in all regulatory aspects of this work.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
VB is guarantor of the content of the manuscript, including the data and analysis. The authors confirm that the manuscript has been read and approved by all the named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. The order of authors listed in the manuscript has been approved by all the authors. VB and HAO were involved in study conception and design. VB, PG, TU, LM contributed to acquisition of data. VB, HAO, GD and JJ were involved in analysis and interpretation of data. VB, LB and HAO contributed to drafting of manuscript. JJ, PG, TU, LM, MG, JLB, BG, GD, EM and LB were involved in critical revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the French Intensive Care Society (Commission d’Ethique de la Société de Réanimation de Langue Française, SRLF-CE 22-046) and the Institutional review board (Comité de Protection des Personnes Est; approval MR-004). Written informed consent was obtained from patients.
Consent for publication
Not applicable.
Competing interests
No competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Supplemental Fig 1
: Description of the Pulsar Model 14® Sound Level Meter and method for measurement. The Model 14 is a general purpose digital sound level meter which meets the full requirements of IEC 61672 to Class 2. Before each inclusion the Sound Level Meter was calibrated acoustically using an external reference, i.e the Sound Level Calibrator Model 106, which is placed over the microphone. The calibrator generates a stabilized Sound Pressure Level of 94dB (+- 0.3dB) at a frequency of 1 kHz. Using a Low range (Low = 35dB to 100dB), maximum sound level was measured pressing the MAX HOLD button for at least ½ second and was ultimately noticed. A level of sound in decibels (L) is defined as ten times the base-10 logarithm of the ratio between two power-related quantities I (i.e cough-volume related sound) and Io (i.e the human hearing threshold) as follows: L = 10 * Log 10 (I/ Io). Thus, an apparent mild increase from 73 to 76 dB in sound level results in multiplying acoustic energy by a factor two.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bonny, V., Joffre, J., Gabarre, P. et al. Sonometric assessment of cough predicts extubation failure: SonoWean—a proof-of-concept study. Crit Care 27, 368 (2023). https://doi.org/10.1186/s13054-023-04653-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04653-w