Abstract
Background
Post-market analyses revealed unanticipated links between first-generation Bruton’s tyrosine kinase inhibitor (BTKi) therapy, ibrutinib, and profound early hypertension. Yet, whether this is seen with novel selective second (next)-generation BTKi therapy, acalabrutinib, is unknown.
Methods
Leveraging a large cohort of consecutive B cell cancer patients treated with acalabrutinib from 2014 to 2020, we assessed the incidence and ramifications of new or worsened hypertension [systolic blood pressure (SBP) ≥ 130 mmHg] after acalabrutinib initiation. Secondary endpoints were major cardiovascular events (MACE: arrhythmias, myocardial infarction, stroke, heart failure, cardiac death) and disease progression. Observed incident hypertension rates were compared to Framingham heart-predicted and ibrutinib-related rates. Multivariable regression and survival analysis were used to define factors associated with new/worsened hypertension and MACE, and the relationship between early SBP increase and MACE risk. Further, the effect of standard antihypertensive classes on the prevention of acalabrutinib-related hypertension was assessed.
Results
Overall, from 280 acalabrutinib-treated patients, 48.9% developed new/worsened hypertension over a median of 41 months. The cumulative incidence of new hypertension by 1 year was 53.9%, including 1.7% with high-grade (≥ 3) hypertension. Applying the JNC 8 cutoff BP of ≥ 140/90 mmHg, the observed new hypertension rate was 20.5% at 1 year, > eightfold higher than the Framingham-predicted rate of 2.4% (RR 8.5, P < 0.001), yet 34.1% lower than ibrutinib (12.9 observed-to-expected ratio, P < 0.001). In multivariable regression, prior arrhythmias and Black ancestry were associated with new hypertension (HR 1.63, HR 4.35, P < 0.05). The degree of SBP rise within 1 year of treatment initiation predicted MACE risk (42% HR increase for each + 5 mmHg SBP rise, P < 0.001). No single antihypertensive class prevented worsened acalabrutinib-related hypertension.
Conclusions
Collectively, these data suggest that hypertension may be a class effect of BTKi therapies and precedes major cardiotoxic events.
Similar content being viewed by others
Introduction
Acalabrutinib is a novel selective, second-generation Bruton’s tyrosine kinase (BTK) inhibitor with dramatic efficacy against B cell malignancies [1,2,3,4,5]. Compared to ibrutinib, a first in-class BTK inhibitor, acalabrutinib has lower alternative kinase inhibition and off-target activity [6,7,8]. Due to a high burden of cardiotoxicity, namely the development of new or worsened hypertension or arrhythmias in follow-up, indefinite ibrutinib use has been limited to those without intolerable effects or disease progression [9,10,11,12,13,14,24, 25]. Within this, only those with patients aged 20 to 69 years without a diagnosis of diabetes were included, akin to the originally validated prediction model.24 The observed rates of hypertension were calculated using the cumulative incidence of blood pressure ≥ 140/90 mmHg at 3-month intervals across the initial year of treatment. The Framingham-predicted rate of new hypertension within 1 year was estimated by averaging the individual patient probabilities obtained after applying individual patient-level risk factors. All analyses were performed with R version 3.6.2 and SAS Software version 9.4, and the statistical tests were two-sided with statistical significance evaluated at the α = 0.05 significance level.
Results
Overall, 280 patients treated with acalabrutinib were identified (Additional file 1: Figure S1). The mean age was 63.7 ± 10.1 years (range 20–89 years), and 28.9% of patients were female. Most (89.0%) had chronic lymphocytic leukemia (CLL), and 279 had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Seventy-two patients were previously treated with ibrutinib; 165 (58.9%) had baseline hypertension at the time of acalabrutinib initiation, of which 57% were on at least 1 antihypertensive medication. Additional baseline characteristics are described in Table 1.
Incident hypertension
Over a median follow-up of 41 months (IQR 20–62 months; range 0–76 months), 59.2% developed new or worsened hypertension, utilizing a SBP cutoff of 130 mmHg (Fig. 1; Additional file 1: Table S1), of which 84.3% had at least probable association with acalabrutinib [23]. The mean increase in SBP was + 7.2 (19.7) mmHg, with a median time to maximum SBP increase of 6 months. A ≥ 10 mmHg increase in SBP from baseline was observed in 35% of patients, and a ≥ 20 mmHg increase was observed in 13.5% of patients (Additional file 1: Table S2). Among those without baseline hypertension, 62 patients (53.9%) developed new hypertension after acalabrutinib initiation, wherein the mean increase in SBP (SD) was 16.7 mmHg (24.2), with a median time to maximum SBP increase of 15 months. Within 1 year of follow-up, the mean maximum increase in SBP was 14.4 mmHg, with a median time to maximum increase in SBP of 6 months, in those with new hypertension. This included 82.3% (n = 51) who reached hypertension thresholds within 6 months of acalabrutinib initiation. In those with baseline hypertension, worsened hypertension was noted among 45.5% (n = 75), including 50.7% (n = 38) with an increase in CTCAE hypertension grade. Moreover, among those treated without intervening monoclonal antibody therapy (obinutuzumab, a known blood pressure depressant), the mean increase in SBP (SD) was 6.45 mmHg (20.0) and the mean peak increase in SBP was 10.1 mmHg, with a median time to maximum SBP increase of 6 months (Additional file 1: Table S3). In total, 9 (3.5%, excluding 20 with baseline high-grade hypertension) developed new high-grade (≥ 3) hypertension while on acalabrutinib, including 1.7% of those without baseline hypertension. No patients required dose reduction due to hypertension. One patient required hospitalization for worsened hypertension (and diastolic heart failure).
A. Change in mean blood pressure during acalabrutinib treatment over the 60-month study period; standard deviation represented by error bars. B. Cumulative incidence of new hypertension (HTN) across time following acalabrutinib initiation. C. Cumulative incidence of worsened HTN across time following acalabrutinib initiation
In univariate analysis, BMI > 25, prior arrhythmia, and history of heart failure were associated with new or worsened hypertension (Additional file 1: Table S4). There was no relationship between acalabrutinib dose and new or worsened hypertension development. However, in multivariable modeling, Black ancestry [hazard ratio (HR) 4.35, 95% confidence interval (CI) 1.21–15.63; P = 0.024], prior AF (HR 1.63, 95% CI 1.06–2.49; P = 0.025), and BMI (HR 1.05, 95% CI 1.02–1.09; P = 0.005) remained associated with new or worsened hypertension (Table 2). These effects were consistent even after removing patients previously on ibrutinib to account for possible confounding of prior AF (Additional file 1: Table S5A). Among patients without baseline hypertension, Black ancestry and prior arrhythmias were significantly associated with development of new hypertension (Additional file 1: Table S5B); additionally, there was a trend of association between baseline SBP and new hypertension development (HR 1.75 across baseline SBP strata vs SBP ≥ 120 mmHg; P = 0.126). Among those with baseline hypertension, only age and BMI were associated with worsened hypertension development (Additional file 1: Table S5C).
Effect of standard antihypertensive therapies
The initiation of antihypertensive therapy was required for 43 patients, including 10 without baseline hypertension. Over time, 17 required combination therapy for the management of hypertension after acalabrutinib initiation. The most common treatment was diuretics, followed by calcium channel blockers and beta-blockers. There was no difference in effect of any specific class on blood pressure control (Additional file 1: Table S6A-C). Yet, the initiation of combination therapy was associated with a –3.32 mmHg reduction in SBP over time (Additional file 1: Table S6D).
Relationship of new or worsened hypertension to major cardiovascular events
MACE were observed among 41 patients (14.6%), including 18.2% with new or worsened hypertension. This was compared to 11.2% with MACE among those without new or worsened hypertension after acalabrutinib initiation (Additional file 1: Table S7). Most events, including 62.1% of arrhythmias, were of probable or definite association with acalabrutinib [23]. AF was the most common cardiovascular complication during acalabrutinib use (8.2%), followed by ventricular arrhythmia (2.9%), SVT (2.5%), and heart failure (2.1%); 1 patient had sudden death. In those with MACE, 66.7% had at least two cardiac risk factors. There was no clear difference in MACE by new or worsened hypertension status in a multivariable model containing known predictors of MACE (HR 1.12, P = 0.751; Table 3; Additional file 1: Table S8A-B). However, the magnitude of early SBP increases within 1 year of acalabrutinib initiation related to the risk of subsequent AF (P < 0.001; Additional file 1: Figure S2), as well as MACE (P < 0.001; Fig. 2). For every 5 mmHg SBP increase, there was a 27% (0.27) increase in MACE risk (P < 0.001), and a 42% (0.42) increase in the risk for AF development (P < 0.001). Among those with new or worsened hypertension, there was no difference in MACE risk by antihypertensive initiation status. Further, in landmark analysis restricted to those free of disease progression with continued acalabrutinib use beyond 3 months, there was no difference in progression-free survival or mortality by new or worsened hypertension status (Additional file 1: Figure S3).
A Cumulative incidence of major cardiovascular events (MACE) across time following acalabrutinib initiation. B. Risk of develo** MACE defined by hazard ratio (HR) stratified by peak systolic blood pressure (SBP) increase within 1 year of acalabrutinib initiation. Dotted line represents hazard ratio = 1. *Asterisks denote statistical tests that did not converge or strata with ≤ 3 patients
Comparative incidence of hypertension with acalabrutinib
Moreover, in those without baseline hypertension, 24.4% developed new hypertension using older JNC-8 cutoffs for hypertension classification. Among those patients aged 20 to 69 years without a diagnosis of diabetes (n = 83), the cumulative incidence of at hypertension at 1 year was 20.5%. This translated into an observed new hypertension cumulative incidence of 205 per 1,000 person-years. Compared to the Framingham risk predicted rate of 24 per 1,000 person-years, this translated into an observed-to-expected ratio of 8.5 (P < 0.001; Fig. 3). Yet, when compared to the previously reported Framingham-adjusted new hypertension rate at 1 year of 312 per 1,000 person-years [11] (12.9 observed-to-expected ratio), this translated into a relative risk reduction of 34.1% for acalabrutinib vs. ibrutinib for incident hypertension (RR 0.66, P < 0.001; Additional file 1: Table S9).
A. Observed versus predicted cumulative incidence of new hypertension (HTN) rates at 1 year, including population and Framingham risk-adjusted rate for ibrutinib [11, 24]. Reflects the JNC-8 HTN cutoff of 140/90 mmHg, in comparison with established HTN prediction models.24 Subjects without known discussion of parenteal history of HTN were assigned a value of 1 (i.e., one of two parents with HTN) in the Framingham model B. Observed versus predicted cumulative incidence of new HTN, including incidence of grade 3 or higher HTN, at 0, 3, 6, 9, and 12 months
Discussion
In this evaluation of the incidence, risk factors, and ramifications of hypertension after acalabrutinib initiation, nearly 50% of patients developed new or worsened hypertension within 1 year of treatment initiation. Outside of Black ancestry and BMI, there are no other factors associated with the development of incident acalabrutinib-related hypertension. Although the rate of hypertension was markedly lower than observed with ibrutinib, the adjusted incidence was still over eightfold higher than predicted at 1 year. This relationship remained even after accounting for age and the burden of traditional cardiovascular risks. In those with early SBP elevation, the rate of other MACE was elevated. Further, blood pressure elevation control frequently required combinational therapy. Nevertheless, no antihypertensive class clearly prevented worsening hypertension. Given the growing use of second-generation BTK inhibitors, and the lack of real-world data to inform their use, these data may have important ramifications on the interpretation and management of cardiovascular risk in patients treated with these emerging therapies.
The observation of increased hypertension with acalabrutinib adds to a growing body of evidence linking BTK inhibition with blood pressure modulation. In an evaluation of ibrutinib-treated patients, initiation of ibrutinib is associated with a 71% incidence of new hypertension after treatment initiation [11]. Similarly, in a separate multicenter evaluation, ibrutinib associated with a median increase of > 13 mmHg in SBP measures within months of treatment initiation [26]. In acalabrutinib-focused studies, secondary analyses suggest that up to 40% of patients may experience increase in hypertension grade or see new hypertension [4]. Although landmark trials, including the ACE-CL-001 and ELEVATE-RR trials, have attempted to evaluate the incidence of hypertension as a secondary outcome, due to the only more recent emergence of the relevance of hypertension with BTK inhibitor therapy, without untreated controls, the measures, timing, and implementation of contemporary blood pressure definitions were not well established [1, 11, 18, 27, 28]. Furthermore, due to potential confounding nature of comorbid risk factors, understanding the true effects of therapy has remained challenging [27]. Within this study, we observed that acalabrutinib treatment results in increased rate of incident hypertension, even after accounting for confounding traditional risk factors. The establishment of these effects provides a key basis for the assessment of the vascular effects of next-generation BTK inhibition among B cell patients treated with these emerging therapies.
Blood pressure elevation is often underappreciated clinically in patients presenting for ongoing anticancer therapies [28, 29]. Due to the confounding nature of blood pressure elevation in cancer populations, particularly at times of increased stress, potential fear of negative news, and necessary focus on potentially fatal disease control, blood pressure elevations may be less well recognized in clinical care settings. However, increasingly accelerated hypertension has been noted to contribute to the unanticipated higher burden of cardiovascular events among patients treated with BTK inhibitors and other anticancer therapies [11, 28]. Blood pressure increases appeared broader and more pronounced with acalabrutinib treatment in the ASCEND trial than the standard therapy arm [30]. This was also clearly observed in the ACE-CL-001 and ACE-CL-003 phase 1b/2 studies [1, 4]. Outside of the current evaluation, post-marketing evaluations of the effects of acalabrutinib or other next-generation BTK inhibitor therapies are largely unavailable. Given the potential for differential observations after clinical dissemination in more focused investigation, and the unintended but serious consequences of cardiotoxicity, enhanced surveillance may prove beneficial.
Although reasons for these observations are not clear, acalabrutinib has reduced inhibition of alternative kinases inclusive of epidermal growth factor receptor (EGFR), extracellular signal-regulated kinase (ERK), and interleukin 2-inducible T cell kinase (ITK) in preclinical models compared to ibrutinib [1, 6,7,8]. Furthermore, in a recent preclinical AF model, acalabrutinib did not share activity with the C-terminal Src kinase (CSK), a pathway linked with AF in mice receiving ibrutinib therapy [15]. Conversely, as with ibrutinib, acalabrutinib may share indirect nitric oxide inhibition [31]. Despite differences in the degree of activation, downstream remodeling inclusive of vasoconstriction and replacement fibrosis have been postulated [11, 27]. These alterations have been recognized to drive disproportionate manifestations of cardiovascular disease in other populations [32,33,34]. However, with BTK inhibitors, elucidation of these pathways may require additional mechanistic and prospective studies.
Limitations
Several limitations should be acknowledged. Due to the retrospective nature of this study, and prevalent BTKi use, no cancer-specific control was available. Follow-up was non-uniform. Similarly, the approach and timing of blood pressure acquisition were non-uniform. While antihypertensives were frequently employed in those with higher elevation, the timing and decision to initiate antihypertensive therapy was at the discretion of treating clinicians. We could not fully determine the effect of antihypertensive therapies on MACE risk or blood pressure control due to variability in treatment regimens. Similarly, the selected class and dose of therapy was not predetermined. However, the low incidence of acalabrutinib discontinuation suggests at least some efficacy of combinational standard antihypertensive care. Although we adjusted for multiple factors, it is possible that the presence of cancer increases hypertension risk [27]. Moreover, some out-of-hospital cardiac events may have gone uncaptured, despite extensive search.
Conclusions
Patients treated with BTK inhibitors face an increased risk of cardiovascular sequelae. Treatment with acalabrutinib associates with significantly elevated risk of early onset hypertension, even after accounting for traditional risk factors. However, the degree of this hypertension is reduced compared to ibrutinib. Given the anticipated increase in acalabrutinib use, further studies evaluating the mechanisms and optimal management strategies for hypertension are needed.
Availability of data and materials
For original data, please contact daniel.addison@osumc.edu.
Abbreviations
- BTK:
-
Bruton’s tyrosine kinase
- SBP:
-
Systolic blood pressure
- SPRINT:
-
Systolic blood pressure interventional trial
- CTCAE:
-
Common terminology criteria for adverse events
- MACE:
-
Major cardiovascular events
- AF:
-
Atrial fibrillation
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- HR:
-
Hazard ratio
- CKD:
-
Chronic kidney disease
- BMI:
-
Body mass index
- CLL:
-
Chronic lymphocytic leukemia
- ECOG:
-
Eastern Cooperative Oncology Group
- CI:
-
Confidence interval
- EGFR:
-
Epidermal growth factor receptor
- ERK:
-
Extracellular signal-regulated kinase
- ITK:
-
Interleukin 2-inducible T cell kinase
- CSK:
-
C-terminal Src kinase
References
Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic Leukemia. N Engl J Med. 2016;374(4):323–32.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. The Lancet. 2018;391(10121):659–67.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial. The Lancet. 2020;395(10232):1278–91.
Woyach JA, Blachly JS, Rogers KA, Bhat SA, Jianfar M, Lozanski G, et al. Acalabrutinib plus Obinutuzumab in treatment-Naïve and relapsed/refractory chronic lymphocytic Leukemia. Cancer Discov. 2020;10(3):394–405.
Byrd JC, Woyach JA, Furman RR, Martin P, O’Brien S, Brown JR, et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021;137(24):3327–38.
Patel V, Balakrishnan K, Bibikova E, Ayres M, Keating MJ, Wierda WG, et al. Comparison of acalabrutinib, a selective bruton tyrosine kinase inhibitor, with ibrutinib in chronic lymphocytic Leukemia cells. Clin Cancer Res. 2017;23(14):3734.
Herman SEM, Montraveta A, Niemann CU, Mora-Jensen H, Gulrajani M, Krantz F, et al. The bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic Leukemia. Clin Cancer Res. 2017;23(11):2831.
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): A covalent bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240–52.
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-Rituximab or chemoimmunotherapy for chronic lymphocytic Leukemia. N Engl J Med. 2019;381(5):432–43.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28.
Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–28.
Salem J-E, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, et al. Cardiovascular toxicities associated with Ibrutinib. J Am Coll Cardiol. 2019;74(13):1667–78.
Wiczer TE, Levine LB, Brumbaugh J, Coggins J, Zhao Q, Ruppert AS, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1(20):1739–48.
Guha A, Derbala MH, Zhao Q, Wiczer TE, Woyach JA, Byrd JC, et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72(6):697–8.
**ao L, Salem J-E, Clauss S, Hanley A, Bapat A, Hulsmans M, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src Kinase. Circulation. 2020;142(25):2443–55.
Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6(1): e001049.
Furman RR, Byrd JC, Owen RG, O’Brien SM, Brown JR, Hillmen P, et al. Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in mature B-cell malignancies. Leukemia. 2021;35(11):3201–11.
Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic Leukemia: Results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52.
The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2018;138(17):e426–83.
Division of Cancer Treatment and Diagnosis. Common terminology criteria for adverse events (CTCAE), version 5.0 [Internet]. Bethesda (MD): National Cancer Institute; 2017 [cited 2021 May 19]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: A report from the American heart association. Circulation. 2020;141(9):e139-596.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, et al. A risk score for predicting near-term incidence of hypertension: the Framingham heart study. Ann Intern Med. 2008;148(2):102–10.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311(5):507–20.
Roeker LE, Sarraf Yazdy M, Rhodes J, Goodfriend J, Narkhede M, Carver J, et al. Hypertension in patients treated with Ibrutinib for chronic lymphocytic Leukemia. JAMA Netw Open. 2019;2(12): e1916326.
van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, et al. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128(7):1040–61.
Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncology. 2019;1(2):238–51.
Lee DH, Hawk F, Seok K, Gliksman M, Emole J, Rhea IB, et al. Association between ibrutinib treatment and hypertension. Heart [Internet]. 2021 [cited 2021 Nov 24]; Available from: https://heart.bmj.com/content/early/2021/06/30/heartjnl-2021-319110
Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic Leukemia. J Clin Oncol. 2020;38(25):2849–61.
Natarajan G, Terrazas C, Oghumu S, Varikuti S, Dubovsky JA, Byrd JC, et al. Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. Oncoimmunology. 2016;5(1): e1057385.
Macedo FN, Mesquita TRR, Melo VU, Mota MM, Silva TLTB, Santana MN, et al. Increased nitric oxide bioavailability and decreased sympathetic modulation are involved in vascular adjustments induced by low-intensity resistance training. Front Physiol. 2016;7:265.
Ding N, Yang C, Ballew SH, Kalbaugh CA, McEvoy JW, Salameh M, et al. Fibrosis and inflammatory markers and long-term risk of peripheral artery disease: The atherosclerosis risk in communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2020;40(9):2322–31.
Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–906.
Acknowledgements
The authors acknowledge and thank the patients and their families treated at the Ohio State University Comprehensive Cancer Center.
Funding
This work was supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants K23-CA178183 (Woyach) and R01-CA197870 (Byrd and Woyach), R35-CA197734 (Byrd), K12-CA133250 (Addison and Byrd), and K23-HL155890 (Addison). Dr. Rogers was supported by a scholar in clinical research grant from the Leukemia & Lymphoma Society (CDP 2331–20). Dr. Addison was also supported by a Robert Wood Johnson Foundation (Harold Amos), American Heart Association Program grant. Support was also received from the D. Warren Brown Foundation, Four Winds Foundations, and the Connie Brown CLL Foundation.
The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All of the authors had full access to all of the data in the study, reviewed drafts of the manuscript, and approved the final version. STC, LA, SAB, and DA were responsible for the concept and design of the study; STC, LA, LR, QZ, MP, JG, OK, PR, SK, SAB, and DA acquired, analyzed, or interpreted the data; STC, LA, and DA drafted the manuscript; STC, QZ, and MP performed the statistical analyses; JCB, JW, SAB, and DA provided administrative, technical, or material support; SAB and DA supervised the work; and all other authors were involved in the critical revision of the manuscript for important intellectual content. MG served on the Consulting and Serving on Data Safety and Monitoring Committee for Ascerta related to acalabrutinib. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. JCB has received research and has consulted for Acerta Pharma and Pharmacyclics, Inc. FA has received research funding from Innate Pharma and Pharmacyclics and provided consulting services to Gilead Sciences, Pharmacyclics, Inc., Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology, and served on the speakers bureau of Abbvie and AstraZeneca. KR received research funding from Genentech and participated in an advisor board for Acerta Pharma. AK has provided consulting services for Abbvie, Beigene, Bristol Myers Squibb, and Janssen. MG has provided consulting services for Ascerta and Pharmacyclics. JW received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys and has consulted for Janssen and Pharmacyclics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
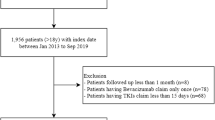
: Table S1 Long-term rates, time to development, and management of new or worsened hypertension (HTN) during acalabrutinib therapy. Table S2 Distribution of maximum SBP increase from baseline, %. Table S3 Development of new or worsened hypertension among patients based on concomitant obinutuzumab treatment. Table S4 Univariable predictors for the development of new or worsened hypertension (n=280). Table S5A Multivariable predictors for the development of new or worsened hypertension, in patients not previously treated with ibrutinib (n=208). Table S5B Multivariable predictors for new hypertension alone (n=115).* Table S5C Multivariable predictors for worsened hypertension alone (n=165).*Table S6A Univariate analysis of association of single-agent baseline antihypertensive therapy (n=115) to development of worsening hypertension (HTN), excluding those on other anti-HTN medications (n=1‡). Table S6B Multivariate analysis of development of worsening hypertension (HTN), considering acalabrutinib users on single-agent baseline beta blocker therapy. Table S6C Multivariate analysis of development of worsening hypertension (HTN), considering acalabrutinib users on any single-agent baseline antihypertensive therapy. Table S6D Change in blood pressure among subjects requiring the addition of new or additional antihypertensive class within the 1st year of acalabrutinib therapy. [From the 43 patients started on a new or additional antihypertensive, 17 patients saw the addition within 12 months of acalabrutinib initiation, of which 10 were treated with the addition of a single antihypertensive drug, 9 had pre- and post-antihypertensive blood pressures; another 7 (out of 17) required initiation of ≥ 2 antihypertensives and had available blood pressure measures pre- and 12 months post-initiation of the first antihypertensive added during acalabrutinib use.] Table S7 Occurrence of major adverse cardiovascular events (MACE), by acalabrutinib-related hypertension (HTN) status. Table S8A Multivariable analysis for the development of MACE during acalabrutinib use, considering development of new or worsened HTN as a time-dependent covariate.*Table S8B Multivariable analysis for the development of AF during acalabrutinib use, considering development of new or worsened HTN as a non-time-dependent covariate. Table S9 Cumulative incidence of new, predicted, and grade 3 or more HTN over time. Figure S1 Study Cohort Diagram. From a registry of all patients with hematologic malignancies treated with acalabrutinib over a 6-year period, those with available blood pressures were included. HTN, hypertension. Figure S2. Risk of AF development, in relationship of observed peak SBP increase within 12 months of acalabrutinib initiation. AF, atrial fibrillation; SBP, systolic blood pressure. Figure S3. Cumulative incidence of disease progression or death among those remaining on acalabrutinib beyond 90 days (landmark) alone, without initial progression or death, by new or worsened hypertension status.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S.T., Azali, L., Rosen, L. et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J Hematol Oncol 15, 92 (2022). https://doi.org/10.1186/s13045-022-01302-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-022-01302-7