Abstract
Background
The impact of sex on prognosis of patients with esophageal squamous cell cancer (ESCC) who underwent definitive radiotherapy remained unclear. The present study aimed to determine the impact of sex on the prognosis of patients with ESCC underwent definitive radiotherapy.
Methods
Between January 2009 and December 2015, patients with ESCC underwent definitive radiotherapy in Shantou Central Hospital were included in this study. The Progression-free survival (PFS) and overall survival (OS) rates were estimated using the Kaplan-Meier method. The PFS and OS were compared between female and male patients. The Cox regression model was used to identify prognostic factors. Propensity score-matched analysis was performed to balance baseline characteristics between female and male patients.
Results
A total of 683 ESCC patients treated with definitive radiotherapy were included, with 497 male and 186 female patients. In the whole cohort, female patients had a significantly longer median PFS (14.0 months vs 10.6 months, P = 0.0001, HR = 0.688, 95% CI, 0.567–0.836) and OS (20.8 months vs 15.9 months, P = 0.0005, HR = 0.702, 95% CI, 0.575–0.857). In the matched cohort, female patients still had a significantly longer median PFS (13.5 months vs 11.6 months) and OS (19.6 months vs 16.1 months). Multivariate analysis showed that sex was an independent prognostic factor for PFS (HR = 0.746, 95% CI, 0.611–0.910, P = 0.004) and OS (HR = 0.755, 95% CI, 0.615–0.926, P = 0.007).
Conclusions
This present study indicated that sex was an independent prognostic factor in Chinese patients with ESCC underwent definitive radiotherapy, with better survival outcome for women than men. Efforts should be made to investigate the underlying biological mechanism.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors in the world with an estimated 477,900 new cases and the fourth most common cause of cancer-related deaths with 375,000 deaths annually [1]. The incidence of EC significantly vary between men and women, with three to five times more prevalent in men than women [2, Follow-up All patients were examined daily during radiotherapy to monitor the treatment toxicities. The first follow-up was 1 month after completing radiotherapy then every 3–6 months for 5 years. The follow-up evaluation included a physical examination, blood test, barium esophagogram, CT scan of the neck, chest, and abdomen, and PET-CT when available. Information on patients’ age, gender, work-up, treatment, and follow-up was extracted from their medical records. Progression was defined as the radiographic evidence of relapse, death from any cause. OS was measured from the date of treatment to the date of death from any cause or last follow-up. Comparisons of patient characteristics were carried out with a chi-square test. PFS and OS rates were estimated by the Kaplan-Meier method, and log-rank test was performed to evaluate the survival difference. The Cox regression model was used to perform multivariate analysis to identify prognostic factors. The propensity score-matched analysis (including age, tumor location, T stage, N stage, TNM stage, Treatment modality (definitive radiotherapy or definitive chemoradiotherpy), and radiotherapy dose) was performed using the one-to-one nearest neighbor method. A P value < 0.05 indicated a statistical significance. All P values were two-sided. The statistical software IBM SPSS v22.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.Statistical analysis
Results
Patient characteristics
Between January 2009 and December 2015, a total of 683 patients with ESCC underwent definitive radiotherapy were included in this study, with 497 men and 186 women Additional file 2. All the patients comleted the thereapy. Patient characteristics were summarized in Table 1, and the distribution of age, T stage, N stage, TNM stage and treatment modality between men and women patients were unbalanced. The baseline characteristics were comparative after propensity score-matching (Table 1). Finally, 178 men patients and 178 women patients were included in the propensity score-matched cohort.
Sex and survival for ESCC
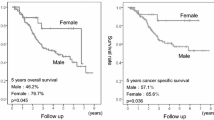
Median follow-up was 16.6 months (range, 1.6 to 112.5 months) in the whole cohort, with a median follow-up of 15.9 months (range, 1.6 to 112.5 months) for men and 21.1 months (range, 1.8 to 111.3 months) for women. In the whole cohort, women had a significantly longer median PFS time compared with men patients (14.0 months vs 10.6 months, χ2 = 14.202, P = 0.0001, HR = 0.688, 95% CI, 0.567–0.836, Fig. 1a). The 3-and 5-year PFS rates were 33.2 and 26.3% in women and 22.0 and 15.7% in men, respectively. The median OS time was 20.8 months in women, which was significantly longer than 15.9 months in men (χ2 = 12.138, P = 0.0005, HR = 0.702, 95% CI, 0.575–0.857, Fig. 1b). The 3-and 5-year OS rates were 36.7 and 31.5% in women and 26.9 and 17.7% in men, respectively.
These findings were further confirmed in the matched cohortThe median PFS times were 13.5 months (95% CI, 11.436–15.564 months) for women and 11.6 months (95% CI, 8.259–14.941 months) for men (χ2 = 5.910, P = 0.015, HR = 0.751, 95% CI, 0.595–0.947, Fig. 2a). The median OS time were 19.6 months (95% CI, 13.571–25.629 months) and 16.1 months (95% CI, 13.776–18.424 months) in women patients and men (χ2 = 6.741, P = 0.0094, HR = 0.733, 95% CI, 0.578–0.82, Fig. 2b), respectively.
Sex and prognostic factors for ESCC
Multivariate analysis showed that sex (HR = 0.746, 95% CI, 0.611–0.910, P = 0.004), tumor location (HR = 1.162, 95% CI, 1.031–1.308, P = 0.014), T stage (HR = 1.184, 95% CI, 1.029–1.362, P = 0.019), N stage (HR = 1.328, 95% CI, 1.013–1.741, P = 0.040), TNM stage (HR = 1.236, 95% CI, 1.014–1.506, P = 0.036) and treatment modality (HR = 0.741, 95% CI, 0.621–0.884, P = 0.001) were independent prognostic factors for PFS (Table 2 and Additional file 1: Table S1), and identified sex (HR = 0.755, 95% CI, 0.615–0.926, P = 0.007) as an independent prognostic factor for OS, as well as tumor location (HR = 1.131, 95% CI, 1.003–1.275, P = 0.045), T stage (HR = 1.266, 95% CI, 1.095–1.462, P = 0.001), RT dose (HR = 0.970, 95% CI, 0.946–0.995, P = 0.017), and treatment modality (HR = 0.712, 95% CI, 0.595–0.852, P = 0.000).
Sex and recurrence for ESCC
As shown in Fig. 3a, after a median follow-up of 16.6 months (range, 1.6 to 112.5 months), the logoregional recurrence rate in female patients was significantly lower than that in men (χ2 = 11.11, P = 0.0009). A similar finding was observed for distant metastasis event, with fewer patients experiencing distant metastases in female group than in male group (χ2 = 7.446, P = 0.0064, Fig. 3b).
Discussion
ESCC and EAC, the two major histologic subtypes of EC, were different in terms of geographic and demographic characteristics, risk factors, and pathogenesis [15]. ESCC, the predominant subtype of EC, was a great health burden in China due to its poor prognosis [1, 16, 17]. For patients with ESCC who refused surgery or patients with a unresectable disease, definitive radiotherapy or chemo-radiotherapy was the standard treatment [18]. However, most patients treated with definitive chemo-radiotherapy relapsed and finally succumbed to this disease [16, 19, 20]. Therefore, identifying patients who were most likely to benefit from definitive radiotherapy would help to investigate the biological mechanisms affecting the radio-sensitivity of ESCC.
According to statistics, for all cancers combined, women had better survival than men in China [1]. Several studies had evaluated the influence of sex on prognosis in patients with EC [8, 10,11,12, 21]. Bohanes et al [8] reported the largest register-based study based on the Surveillance, Epidemiology, and End Results (SEER) database and demonstrated that women had better survival in loco-regional ESCC but not in EAC. Another register-based study from Sweden indicated that women had a lower overall all-cause mortality compared with men only in ESCC [11]. Both two studies did not report detailed clinical information about treatment modality. For patients who underwent surgery, women with ESCC were found to have better survival than men in several studies [10, 21]. However, influence of sex on the patients with ESCC who underwent definitive radiotherapy was inadequately studied. In the current study, women with ESCC who underwent definitive radiotherapy had a better PFS and OS than men, and multivariate analysis suggested sex was an independent prognostic factor. Moreover, women had lower overall recurrence and distant metastases. In addition, after balancing clinicopathological prognostic factors including age, tumor location, T stage, N stage, TNM stage, radiotherapy dose, and treatment modality by propensity score matching, better PFS and OS remained in female patients. Taken together, female patients who underwent definitive radiotherapy for ESCC had better prognosis than men.
However, the prognostic significance of sex in ESCC should be interpreted cautiously. Zhang et al. assessed the role of sex on prognosis of ESCC in Chinese and in Caucasian patients in the United States, and found that sex was not an independent prognostic factor in these patients [22]. However, that report didn’t provide specific information about variables and treatment modality which might affect the analysis. In a post hoc analysis that examined the role of sex in Asian patients with ESCC [22], no significant difference in esophageal cancer-specific survival was observed between women and men. Therefore, the role of sex on prognosis of ESCC could not be generalized to different ethnic groups and patients who received different treatments. In the current study, the significance of sex in ESCC was evaluated in the patients who underwent definitive radiotherapy in ChaoShan region in Guangdong Province, China, adding evidence to the effects of sex on the prognosis of ESCC.
Another significant difference observed in the current study was that more male patients experienced regional recurrence and distant metastasis than female patients, indicating a better disease control after radiotherapy in women. However, whether sex affected the radio-sensitivity of ESCC was unclear, a direct influence of sex on radiation sensitivity in ESCC were under-investigated. A retrospective study of patients treated with neoadjuvant chemoradiotherapy (nCRT) followed by surgery may explained the different prognosis between female and male patients treated with definitive radiotherapy [12]. After nCRT, more women (58%) attained a complete or nearly pathologic response compared with men (47%). Moreover, men had an 80% increase in the risk of recurrence. The merits of our study were its sample size and its assessment for the direct influence of sex on prognosis of patients treated with definitive radiotherapy, and identified sex as an indicator of radio-sensitivity to radiotherapy in ESCC.
On the basis of our current data, we hypothesized that sex difference would affect the radio-sensitivity of patients with ESCC exposed to radiotherapy. Following efforts will be made to find out the relation between sex and radio-sensitivity in ESCC. Previous studies have shown that androgen exposure can facilitate the growth of human esophageal squamous cell carcinoma cells and activation of androgen receptors may promote progression of esophageal squamous cell carcinoma [23, 24]. The relation between androgen levels and prognosis of patients with ESCC exposed to radiotherapy will be investigated in our further study. Some experiments in vivo and vitro will be performed to explore the mechanism how androgen affects the radio-sensitivity of esophageal squamous cell carcinoma. Once confirmed, some future clinical research comparing combined chemoradiotherapy and anti-androgenic treatment with chemoradiotherapy alone in ESCC may be considered. Speculatively, intensified chemoradiotherapy would be warranted and anti-androgenic treatment may be added to chemoradiotherapy in male patients with ESCC. But for now, to be honest, evidence was still weak. Consolidation chemotherapy after definitive CRT might be a strategy for male patients.
The current study had several limitations. The primary limitation was that the information about lifestyle factors, socioeconomic status, radiotherapy target, and other prognostic risks was not available. In addition, the retrospective and nonrandomized design limited the strength of the results.
Conclusion
Our study demonstrated that sex was an independent prognostic factor in Chinese patients with ESCC who underwent definitive radiotherapy, with a better prognosis in female patients than men patients. Efforts should be made to investigate the biological mechanisms for the sex difference in the prognosis of ESCC patients.
Abbreviations
- CTV:
-
Clinical target volume
- CTVnd:
-
nodal clinical target volume
- CTVt:
-
tumor clinical target volume
- EAC:
-
Esophageal adenocarcinoma
- EC:
-
Esophageal cancer
- ESCC:
-
Esophageal squamous cell cancer
- GTV:
-
Gross tumor volume
- GTVnd:
-
nodal gross tumor volume
- nCRT:
-
neoadjuvant chemoradiotherapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PTV:
-
Planning target volume
- SEER:
-
Surveillance, Epidemiology, and End Results
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin. 2016;66(2):115–32.
Jemal A, Bray F, Center MM, et al. Global cancer statistics [J]. CA Cancer J Clin. 2011;61(2):69–90.
**e SH, Lagergren J. A global assessment of the male predominance in esophageal adenocarcinoma [J]. Oncotarget. 2016;7(25):38876–83.
Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis [J]. Am J Gastroenterol. 2014;109(6):822–7.
Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma [J]. CA Cancer J Clin. 2013;63(4):232–48.
Versteijne E, van Laarhoven HW, van Hooft JE, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern [J]. Dis Esophagus. 2015;28(5):453–9.
Jeene PM, Versteijne E, van Berge Henegouwen MI, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation [J]. Acta Oncol. 2017;56(1):33–8.
Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer [J]. J Clin Oncol. 2012;30(18):2265–72.
Rohatgi PR, Correa AM, Swisher SG, et al. Gender-based analysis of esophageal cancer patients undergoing preoperative chemoradiation: differences in presentation and therapy outcome [J]. Dis Esophagus. 2006;19(3):152–7.
Morita M, Otsu H, Kawano H, et al. Gender differences in prognosis after esophagectomy for esophageal cancer [J]. Surg Today. 2014;44(3):505–12.
Kauppila JH, Wahlin K, Lagergren P, Lagergren J. Sex differences in the prognosis after surgery for esophageal squamous cell carcinoma and adenocarcinoma[J]. Int J Cancer. 2019;144(6):1284–91.
Rowse PG, Jaroszewski DE, Thomas M, et al. Sex Disparities After Induction Chemoradiotherapy and Esophagogastrectomy for Esophageal Cancer [J]. Ann Thorac Surg. 2017;104(4):1147–52.
Kauppila JH, Mattsson F, Brusselaers N, et al. Prognosis of oesophageal adenocarcinoma and squamous cell carcinoma following surgery and no surgery in a nationwide Swedish cohort study [J]. BMJ Open. 2018;8(5):e021495.
Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma [J]. Ann Thorac Surg. 2010;89(4):1024–31.
Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer [J]. Lancet. 2017;390(10110):2383–96.
Chen H, Zhou L, Yang Y, et al. Clinical Effect of Radiotherapy Combined with Chemotherapy for Non-Surgical Treatment of the Esophageal Squamous Cell Carcinoma [J]. Med Sci Monit. 2018;24:4183–91.
Chen WQ, Li H, Sun KX, et al. Report of Cancer Incidence and Mortality in China, 2014 J. Zhonghua Zhong Liu Za Zhi. 2018;40(1):5–13.
Sohda M, Kuwano H. Current Status and Future Prospects for Esophageal Cancer Treatment [J]. Ann Thorac Cardiovasc Surg. 2017;23(1):1–11.
Cao CN, Luo JW, Gao L, et al. Intensity-modulated radiotherapy for cervical esophageal squamous cell carcinoma: clinical outcomes and patterns of failure [J]. Eur Arch Otorhinolaryngol. 2016;273(3):741–7.
Brower JV, Chen S, Bassetti MF, et al. Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012[J]. Int J Radiat Oncol Biol Phys. 2016;96(5):985–93.
Hidaka H, Hotokezaka M, Nakashima S, et al. Sex difference in survival of patients treated by surgical resection for esophageal cancer [J]. World J Surg. 2007;31(10):1982–7.
Zhang J, Garfield D, Jiang Y, et al. Does sex affect survival of patients with squamous cell esophageal cancer?[J]. J Clin Oncol. 2013;31(6):815–6.
Matsuoka H, Sugimachi K, Ueo H, et al. Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma [J]. Cancer Res. 1987;47(15):4134–40.
Dong H, Xu J, Li W, et al. Reciprocal androgen receptor/interleukin-6 crosstalk drives oesophageal carcinoma progression and contributes to patient prognosis [J]. J Pathol. 2017;241(4):448–62.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
H-CH and L-XL designed the study. H-SL prepared figures and wrote the manuscript text. H-YX and S-XW collected the follow-up data. X-YL and Z-SD made statistical analysis. All authors reviewed the manuscript and approved the final manuscript. H-SL and H-YX contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Multivariate analysis of clinical factors associated with Progression-Free Survival and Overall Survival among patients with ESCC. (DOC 34 kb)
Additional file 2:
Flow chart of patients selection. (TIFF 32 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Luo, HS., Xu, HY., Du, ZS. et al. Impact of sex on the prognosis of patients with esophageal squamous cell cancer underwent definitive radiotherapy: a propensity score-matched analysis. Radiat Oncol 14, 74 (2019). https://doi.org/10.1186/s13014-019-1278-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-019-1278-0