Abstract
Background
Moderate physical exercise is conducive to the brains of healthy humans and AD patients. Previous reports have suggested that treadmill exercise plays an anti-AD role and improves cognitive ability by promoting amyloid clearance, inhibiting neuronal apoptosis, reducing oxidative stress level, alleviating brain inflammation, and promoting autophagy–lysosome pathway in AD mice. However, few studies have explored the relationships between the ubiquitin–proteasome system and proper exercise in AD. The current study was intended to investigate the mechanism by which the exercise-regulated E3 ubiquitin ligase improves AD.
Methods
Both wild type and APP/PS1 transgenic mice were divided into sedentary (WTC and ADC) and exercise (WTE and ADE) groups (n = 12 for each group). WTE and ADE mice were subjected to treadmill exercise of 12 weeks in order to assess the effect of treadmill running on learning and memory ability, Aβ plaque burden, hyperphosphorylated Tau protein and E3 ubiquitin ligase.
Results
The results indicated that exercise restored learning and memory ability, reduced Aβ plaque areas, inhibited the hyperphosphorylation of Tau protein activated PI3K/Akt/Hsp70 signaling pathway, and improved the function of the ubiquitin–proteasome system (increased UCHL-1 and CHIP levels, decreased BACE1 levels) in APP/PS1 transgenic mice.
Conclusions
These findings suggest that exercise may promote the E3 ubiquitin ligase to clear β-amyloid and hyperphosphorylated Tau by activating the PI3K/Akt signaling pathway in the hippocampus of AD mice, which is efficient in ameliorating pathological phenotypes and improving learning and memory ability.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is a group of age-related progressive neurodegenerative diseases and is the most common form of dementia. The World Alzheimer’s Disease Report 2019 states that there are currently at least 50 million people with dementia worldwide, and by 2050, the number of people with dementia is projected to be 152 million [1], which has major implications for individuals, families, and health care systems. Therefore, it is increasingly important to conduct more research on the prevention and treatment of AD. The main pathological feature of AD is the presence of a large number of senile plaques with extracellular Aβ as the main component in the cerebral cortex and hippocampus senile plaque (SP) and neurofibrillary tangles (NFTs) with hyperphosphorylated Tau (P-Tau) as the main component, leading to severe impairment of neuronal function and synaptic capacity in specific brain regions, cognitive decline and memory impairment [2]. The degradation of intracellular and/or extracellular abnormal proteins is mainly accomplished by the ubiquitin–proteasome system (UPS) to restore protein homeostasis and maintain all cellular functions. Dysfunctional UPS is associated with the accumulation of Aβ in AD and Tau protein hyperphosphorylation, causing proteostasis imbalance.
UPS-related proteins, such as carboxyl terminus of HSC70 interacting protein (CHIP) and ubiquitin carboxyl-terminal esterase-1 (UCHL-1), play important roles in AD, and their abnormal expressions lead to UPS dysfunction in AD patients [3, 4]. CHIP can act as a co-chaperone of heat shock protein 70 (HSP70) that interacts with HSP70 to form a mediated CHIP/HSP complex leading to ubiquitination of misfolded proteins and assisting in the presentation of target proteins to the proteasome for degradation [5]. In animal models of AD, reduced expressions of HSP70 [6] and deletion of CHIP [7] correspondingly lead to accumulation of hyperphosphorylated tau proteins, suggesting that damage to the folding pathway plays a key role in neurodegeneration and accelerates the progression of AD. In addition, Heat shock factor 1 (HSF1) is a key regulator of induced HSP70 expression, and in AD brains, HSF1 activity and protein expressions are reduced [8], suggesting that the upstream factors of HSP70 are inhibited, that a corresponding cascade response exists to regulate abnormal protein folding. Abnormal function of UCHL-1 and inhibition of ubiquitin hydrolytic activity of UCHL-1 in AD mice disrupt the distribution of synaptic proteins increase the spine size of hippocampal neurons, decrease spine density, and exacerbate cognitive impairment [9, 10]. The expression of UCHL-1 is down-regulated in early AD brains [11], and is involved in the degradation of APP and β-secretase 1 (BACE1). The reduced UCHL-1 content leads to impaired APP and BACE1 degradation and increases the amount of BACE1 [12], which results in Aβ overproduction. Recent research shows that the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway is critical to the physiological and pathological conditions of AD [13]. In the hippocampus of AD transgenic mice, there is an abnormal PI3K/Akt signaling pathway with decreased levels of phosphorylation of PI3K and Akt [14] and suppressed expressions of HSP70 [15, 16], accompanied by increased neuronal loss and apoptosis and increased inflammatory response. In neuronal cells, changes in PI3K/Akt pathway activity modulate HSP70 expressions [17], suggesting that the PI3K/Akt signaling pathway regulates HSP70 translocation, and that HSP70 is a downstream factor of the PI3K/Akt signaling pathway.
In recent years, exercise as a healthy lifestyle has reduced the risk of AD to some extent. Numerous studies have shown that long-term regular exercise can reduce Aβ [18] and hyperphosphorylated Tau protein levels [19], improve the cognitive level of AD patients [20], and effectively delay the onset of AD. Moreover, aerobic exercise has been shown to activate the PI3K/Akt signaling pathway in the brain of AD transgenic mice to upregulate HSP70 protein levels [21], thereby exerting a neuroprotective effect. However, the exact mechanism by which exercise regulates the expression of E3 ligases (CHIP, UCHL-1) to affect UPS function is not clear in the AD state, which was why this study was conducted in APP/PS1 mice to find out whether exercise could improve the pathological phenotype of AD by affecting the PI3K/Akt pathway and thus altering the expression of E3 ubiquitin ligase. This research focused on the effects of exercise on cognitive ability, Aβ plaque areas, Tau protein hyperphosphorylation content, and E3 ubiquitin ligase expression levels.
Materials and methods
Animals
Three-month-old male APPswe/PS1dE9 (APP/PS1) double transgenic mice [B6-Tg (PrP- hAPP/h PS1)] and their age- and background-matched wild-type (WT) mice [C57BL/6] were purchased from the BEIJING HFK BIOSCIENCE CO., LTD, (Bei**g, China). The successful modeling of APP/PS1 transgenic mice could be confirmed by the test report (No. ZG-3.5-02). Mice were housed in pairs in cages and had access to food and water ad libitum. The facility environment had controlled light (12:12 h light/dark cycle), relative humidity (50 ± 10%) and temperature (22 ~ 25 °C) under conventional laboratory conditions. APP/PS1 transgenic mice were randomly assigned to two groups (n = 12 for each group): transgenic control (ADC) and transgenic exercise (ADE). Similarly, wild-type littermate mice were randomly divided into two groups (n = 12 for each group): wild-type control (WTC), wild-type exercise (WTE). All experimental procedures were approved by the Laboratory Animal Welfare Ethics Committee of the Institute of Environmental and Operational Medicine. All efforts were made to minimize the number and suffering of animals used in these experiments.
Treadmill exercise protocols
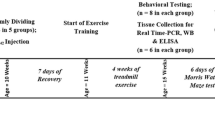
The exercise protocol in this experiment referred to Baker’s research [22] and made appropriate modifications. 45%-55% maximal oxygen uptake (VO2max) was used as the exercise intensity of this experiment, which was an appropriate intensity of long-term aerobic exercise. Mice in WTE and ADE groups received 6-day adaptive training (familiarity period, 15 min/day) to adapt to the new environment. On the first and second days, the treadmill was engaged to a walking speed of 5 m/min before the speed was increased to 8 m/min on the third and fourth days and 12 m/min on the fifth and sixth days. Mice in the exercise groups received formal training on a treadmill with a frequency of 5 days per week for 12 weeks. In each session, mice ran on the treadmill at zero inclination at a speed of 5 m/min for 5 min and then 8 m/min for 5 min (warming up), after which the speed was increased to 12 m/min for 30 min (main exercise), and finally at 12 m/min for 5 min (recovery). Mice assigned to ADC and WTC groups were placed on the static treadmill for a matched stage to minimize the impact of the experimental environment on the results during the entire training (Fig. 1A).
Effect of treadmill exercise on cognitive functions assessed with the Morris water maze (n = 8 for each group). A Schematic diagram of the exercise protocol in mice. B, C Mean escape latencies to reach the platform and average percentage of time spent in the platform quadrant for 5 consecutive days during the navigation test. D, E Numbers of platform crossings and percentage of time spent in the platform quadrant during the probe test on day 6. F, G Percentage of pathlength in the platform quadrant and total swimming distance during the probe test on day 6. Values are presented as mean ± SEM. In the navigation test and probe test, statistically different from WTC, *P < 0.05; **P < 0.01. Statistically different from ADC, #P < 0.05; ##P < 0.01. ADE transgenic exercise, ADC transgenic sedentary, WTE wild-type exercise, WTC wild-type sedentary
Morris water maze test
After the 12-week exercise regimen, the capacity of learning and memory of eight mice from each group was evaluated with a Morris water maze (MWM) test as described previously [23]. Firstly, an appropriate amount of thermostatic water (24 ~ 26 °C) was added to the circular pool, and the height of the water exceeded the platform in the pool by 1–2 cm. The pool was divided into four quadrants and the platform was placed in the first quadrant as the target quadrant. An appropriate amount of skimmed milk powder was sprinkled into the pool to keep the water turbid. Before the initiation of the MWM test, all mice received a 1-day familiarization session to ensure that they could swim and find a round platform. During the place navigation test, the mice were tested for 5 consecutive days (by entering water from fixed positions in quadrant1, 2, 3, and 4) to find the platform (constant position) so as to evaluate the acquisition ability of spatial learning and memory of mice. If the mice failed to find the platform within 60 s, the escape latency was recorded as 60 s, and the mice were placed on the platform to learn for 10–15 s. During the space probe test (day 6), the platform was removed from the maze and the mice were placed in water from four quadrants in sequence to find the location of the platform within 60 s so as to assess their memory retention ability of the platform location. A camera above the pool was used to capture images of the mice swimming during the test. These images were analyzed with a computerized video-tracking system (** AD. Interestingly, our findings found that 12 weeks of treadmill exercise reversed cognitive dysfunction in APP/PS1 transgenic mice (decreased escape latency, increased percentage of time and distance spent in the platform quadrant and numbers of platform crossings) and was accompanied by a reduction in the number of Aβ plaques in the hippocampus. Moreover, we and other studies [41] have also shown that treadmill exercise reduced soluble Aβ peptide levels in the hippocampus.
Impaired UPS function can lead to aggregation and hyperphosphorylation of Tau proteins, and hyperphosphorylated Tau proteins in turn inhibit UPS function [42, 43]. Our study found that phosphorylation levels of Tau Ser202 and Thr181 sites were significantly elevated in the hippocampus of APP/PS1 transgenic mice, while p-Tau levels were significantly reduced in the hippocampal region of AD transgenic mice after 12 weeks of mandatory exercise. This suggested that exercise reduced p-Tau levels and improved the pathological state of AD. The current findings conform with previous studies in which treadmill running suppressed tau phosphorylation levels at Ser404, Ser202, and Thr231 residues in the hippocampus of AD mice [21]. On the other hand, 3 months of treadmill exercise inhibited the expression of P-tau protein at Ser202, Ser404, Ser396 and Thr231 sites in the hippocampus of AD mice by upregulating the expression of phosphorylated PI3K and Akt [19]. Thus, our study also confirmed that exercise activated the PI3K/Akt signaling pathway, reduced p-Tau levels and improved cognitive impairment. The current findings support and extend previous research that physical exercise has a preventive and/or suppressive effects on cognitive decline in AD animal models.
We proceeded to investigate possible signaling pathways involved in the treadmill exercise-induced beneficial effects. Aβ is generated by sequential cleavages of β-amyloid precursor protein (APP) by BACE1 and γ-secretase. Studies have confirmed that as AD worsens, Aβ expression increases and Aβ oligomers inhibit the activation of the PI3K/AKT signaling pathway, leading to a gradual decrease in the phosphorylation expression levels of PI3K subunits and Akt, further causing an increase in BACE1 and PS1 expression, forming a vicious cycle [44]. Our results illustrated that inhibition of the PI3K/Akt signaling pathway in hippocampal tissue of APP/PS1 mice raised BACE1 levels, leading to an increase in the number of Aβ plaques. It was found that treadmill exercise activated the PI3K/Akt pathway in the hippocampus of AD model mice, upregulating p-PI3K and p-Akt levels [45]. After 12 weeks of treadmill training, PI3K p110β and p85α subunit protein levels were significantly upregulated in the hippocampal tissue of the brains of APP/PS1 exercise group mice, and phosphorylation levels at the Akt Ser473 site were also significantly increased, accompanied by a decrease in BACE1 levels. Previous studies have shown that treadmill exercise significantly reduced the expression levels of BACE1 and PS1 in the hippocampal tissue of APP/PS1 mice [46]. In addition, some studies [47] have reported that pharmacological activation of the PI3K/Akt signaling pathway in the cerebral cortex reduces the levels of BACE1 and γ-secretase, thereby reducing the formation of Aβ. The combination of data from different studies implies that treadmill exercise might effectively inhibit Aβ deposition by enhancing PI3K/Akt signaling pathway activity in the hippocampus of AD model mice and reducing the expression level of BACE1.
AD patients have UPS dysfunction. Accumulating evidence indicated that soluble UCHL-1 activity and protein levels are down-regulated and BACE1 levels are upregulated in the brains of postmortem AD patients and in APP/PS1 mouse models [3]. In our study, we also observed significantly elevated gene and protein levels of BACE1 in the hippocampus of APP/PS1 transgenic mice, accompanied by a significant suppression of UCHL-1 protein expression in the AD pathological state. Overexpression of UCHL-1 accelerates BACE1 degradation, impairs APP processing and reduces Aβ production [48]. Interestingly, protein levels of UCHL-1 were significantly increased and accompanied by a decrease in BACE1 levels in the hippocampus of APP/PS1 transgenic mice after exercise. Similarly, treadmill exercise also increased UCHL-1 expression levels in the hippocampus of normal wild-type mice. We speculated that this was possibly because exercise decreased BACE1 levels, leading to a corresponding compensatory increase in UCHL-1 levels. It is unclear whether there is a direct activation relationship between exercise and UCHL-1 or whether there are other factors that influence changes in UCHL-1 levels, and further studies are needed to solve these doubts. With respect to cognitive impairments, UCHL-1 was found to be required for neuronal synapse formation and maintenance of cognitive function. UCHL-1-deficient mice showed decreasing acetylcholine release from the synaptic terminal, reduced ubiquitin recycling and disruption of ubiquitin-dependent pathways, accompanied by hindered synaptic plasticity, nerve terminal retraction and axonal degeneration. Further studies [49] also revealed that overexpression of UCHL-1 repaired situational memory deficits and reestablished synaptic activity in APP/PS1 mice by reducing BACE1 levels. These data suggest that the lower expression of UCHL-1 may be partially responsible for cognitive impairment and AD pathophysiology. Based on our current data, it is at least partially illustrated that (exercise lowering BACE1 levels) may indirectly affect (upregulate) UCHL-1 levels and improve cognitive effect. Therefore, we hypothesized that exercise activated the PI3K/Akt signaling pathway in the hippocampus of APP/PS1 mice, decreased the expression level of BACE1 and correspondingly increased the level of UCHL-1, thereby improving cognitive impairment in the AD state.
Studies have shown that HSF1 and HSP70 are down-regulated in the AD brain [6, 8]. In addition, reduced HSP70 expression in AD brain was also accompanied by a significant decrease in Akt phosphorylation levels [17], suggesting inhibition of the PI3K/Akt signaling pathway in AD brain, increased Aβ misfolding and aggregation, and impaired Aβ clearance. Based on their strong pathological association with AD, we found that mRNA levels of HSF1 and HSP70 and protein content of HSP70 were significantly down-regulated in the hippocampus of APP/PS1 transgenic mice. Early studies have shown that the PI3K/Akt pathway regulates HSF1 gene expression, induces a heat shock response, induces increased HSP70 transcription, inhibits Aβ misfolding and aggregation, protects neuronal cells from stress-induced protein denaturation resulting in damage, and attenuates AD [50]. Another study revealed that exercise activated the PI3K/Akt signaling pathway in the brain of AD mice to upregulate HSP70 expression levels and decrease p-Tau content [21]. In line with this study, exercise was found to significantly increase gene expression of HSF1 and protein content of HSP70 in the hippocampus of APP/PS1 transgenic exercise group mice compared to the ADC group in our study. The above findings implied that exercise activated the PI3K/Akt signaling pathway in the hippocampal tissue of AD mice, upregulated HSF1 and HSP70 gene expression and induced an increase in HSP70 expression, which inhibited the formation of Aβ plaques and reduced hyperphosphorylated Tau protein levels.
To further investigate the specific mechanism of Tau protein dephosphorylation, we focused on another key E3 ligase (CHIP) in the UPS. Previous studies have demonstrated that CHIP deficiency exacerbates the phosphorylation of Tau proteins in the brains of AD mice [7]. Our study also demonstrated a significant decrease in CHIP levels in the hippocampus of APP/PS1 transgenic mice, accompanied by an increase in p-Tau levels. UPS-dependent P-Tau clearance is mediated by the overexpression of HSP70, and increasing the levels or effects of molecular chaperones in the UPS can effectively clear pathogenic proteins [51]. HSP70 acts as a cofactor for CHIP and overexpression of the molecular chaperone HSP70 facilitates clearance of tau by UPS. It has been shown that sulforaphane induces clearance of Aβ and Tau proteins and rescues memory deficits by increasing the expression levels of HSP70 and CHIP in the brains of AD transgenic mice [52]. CHIP binds to HSP70 to form the CHIP–HSP70 complex, which recognizes pathologically phosphorylated Tau and ubiquitinates it, and then passes the ubiquitinated Tau (primarily in its phosphorylated form) to the proteasome for degradation, reducing cell death caused by pathologically phosphorylated Tau protein [53]. Interestingly, we found that exercise significantly increased the protein content of CHIP in the hippocampus of APP/PS1 mice, and we speculated that this was because exercise upregulated HSP70 expression through activation of the PI3K/Akt pathway in the hippocampus of AD mice, which compensated by increasing the protein level of CHIP, which in turn formed a Tau/HSP/CHIP aggregate that was proteasomally degraded, reducing soluble phosphorylated Tau deposition and NFT formation. This also implies that the phosphorylation state of tau is regulated by the Hsp70-based chaperone machinery and undergoes Hsp70/CHIP-dependent ubiquitination and proteasomal degradation.
Conclusion
To conclude, this study demonstrates that 12 weeks of compulsory physical exercise in the early states of AD may serve as a non-pharmacological means to delay and/suppress AD-like cognitive impairments based on MWM, as well as AD progression, by alleviating Aβ plaque burden and Tau hyperphosphorylation levels, promoting UPS function, and reducing Aβ and p-Tau production. The mediation of these effects may involve PI3K/Akt/HSP70 signaling pathway molecules, which also highlights the clinical importance of early interventions in AD patients. Yet, the effect of forced physical exercise on the ubiquitin–proteasome system is not fully understood and a more robust phenotype of AD pathology needs to be established to further explore the exact mechanisms.
Availability of data and materials
Data and materials are available on request.
Abbreviations
- Aβ:
-
Amyloid peptides-β
- AD:
-
Alzheimer’s disease
- ADC:
-
Transgenic sedentary
- ADE:
-
Transgenic exercise
- Akt:
-
Protein kinase B
- APP:
-
Amyloid precursor protein
- BACE1:
-
β-Secretase 1
- CHIP:
-
Carboxyl terminus of HSP70 interacting protein
- HSF1:
-
Heat shock factor 1
- HSP70:
-
Heat shock protein 70
- NFTs:
-
Neurofibrillary tangles
- PI3K:
-
Phosphatidylinositol 3-kinase
- P-Tau:
-
Hyperphosphorylated Tau protein
- SP:
-
Senile plaque
- UCHL-1:
-
Ubiquitin carboxyl-terminal esterase-1
- UPS:
-
Ubiquitin–proteasome system
- WTE:
-
Wild-type exercise
- WTC:
-
Wild-type sedentary
References
Guzman-Martinez L, Maccioni RB, Farias GA, Fuentes P, Navarrete LP. Biomarkers for Alzheimer’s disease. Curr Alzheimer Res. 2019;16:518–28.
Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57:1105–21.
Oddo S. The ubiquitin-proteasome system in Alzheimer’s disease. J Cell Mol Med. 2008;12:363–73.
Bilguvar K, Tyagi NK, Ozkara C, Tuysuz B, Bakircioglu M, Choi M, Delil S, Caglayan AO, Baranoski JF, Erturk O, et al. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:3489–94.
McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–8.
Jiang YQ, Wang XL, Cao XH, Ye ZY, Li L, Cai WQ. Increased heat shock transcription factor 1 in the cerebellum reverses the deficiency of Purkinje cells in Alzheimer’s disease. Brain Res. 2013;1519:105–11.
Dickey CA, Yue M, Lin WL, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AM, et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26:6985–96.
Gomez-Pastor R, Burchfiel ET, Thiele DJ. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:4–19.
Cartier AE, Djakovic SN, Salehi A, Wilson SM, Masliah E, Patrick GN. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci. 2009;29:7857–68.
Graham SH, Liu H. Life and death in the trash heap: the ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia. Ageing Res Rev. 2017;34:30–8.
Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279:13256–64.
Guglielmotto M, Monteleone D, Vasciaveo V, Repetto IE, Manassero G, Tabaton M, Tamagno E. The decrease of Uch-L1 activity is a common mechanism responsible for Abeta 42 accumulation in Alzheimer’s and vascular disease. Front Aging Neurosci. 2017;9:320.
Ali T, Kim T, Rehman SU, Khan MS, Amin FU, Khan M, Ikram M, Kim MO. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55:6076–93.
Zang G, Fang L, Chen L, Wang C. Ameliorative effect of nicergoline on cognitive function through the PI3K/AKT signaling pathway in mouse models of Alzheimer’s disease. Mol Med Rep. 2018;17:7293–300.
Cho JY, Um HS, Kang EB, Cho IH, Kim CH, Cho JS, Hwang DY. The combination of exercise training and alpha-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. Int J Mol Med. 2010;25:337–46.
Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–39.
Sun Y, Zhang JR, Chen S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp Ther Med. 2017;14:5267–74.
Zhao N, Zhang X, Song C, Yang Y, He B, Xu B. The effects of treadmill exercise on autophagy in hippocampus of APP/PS1 transgenic mice. NeuroReport. 2018;29:819–25.
Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561–70.
Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 2019;19:181.
Um HS, Kang EB, Koo JH, Kim HT, ** L, Kim EJ, Yang CH, An GY, Cho IH, Cho JY. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci Res. 2011;69:161–73.
Baker EJ, Gleeson TT. The effects of intensity on the energetics of brief locomotor activity. J Exp Biol. 1999;202:3081–7.
Kim D, Cho J, Lee I, ** Y, Kang H. Exercise attenuates high-fat diet-induced disease progression in 3xTg-AD mice. Med Sci Sports Exerc. 2017;49:676–86.
Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain. 2017;140:3233–51.
Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med (Berl). 2006;84:253–8.
Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–8.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–9.
Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30.
Zhang X, He Q, Huang T, Zhao N, Liang F, Xu B, Chen X, Li T, Bi J. Treadmill exercise decreases abeta deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front Aging Neurosci. 2019;11:78.
Yan QW, Zhao N, **a J, Li BX, Yin LY. Effects of treadmill exercise on mitochondrial fusion and fission in the hippocampus of APP/PS1 mice. Neurosci Lett. 2019;701:84–91.
Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21.
Hashiguchi D, Campos HC, Wuo-Silva R, Faber J, Gomes da Silva S, Coppi AA, Arida RM, Longo BM. Resistance exercise decreases amyloid load and modulates inflammatory responses in the APP/PS1 mouse model for Alzheimer’s disease. J Alzheimers Dis. 2020;73:1525–39.
Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer’s disease. Brain Pathol. 2016;26:62–74.
Chen X, Gan L. An exercise-induced messenger boosts memory in Alzheimer’s disease. Nat Med. 2019;25:20–1.
Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Goncalves RA, Clarke JR, Beckman D, Staniszewski A, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25:165–75.
Kelly AM. Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast. 2018;4:81–94.
Amjad I, Toor H, Niazi IK, Afzal H, Jochumsen M, Shafique M, Allen K, Haavik H, Ahmed T. Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int J Neurosci. 2019;129:551–62.
Yang SY, Shan CL, Qing H, Wang W, Zhu Y, Yin MM, Machado S, Yuan TF, Wu T. The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets. 2015;14:1292–7.
Du Z, Li Y, Li J, Zhou C, Li F, Yang X. Physical activity can improve cognition in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2018;13:1593–603.
Khodadadi D, Gharakhanlou R, Naghdi N, Salimi M, Azimi M, Shahed A, Heysieattalab S. Treadmill exercise ameliorates spatial learning and memory deficits through improving the clearance of peripheral and central amyloid-beta levels. Neurochem Res. 2018;43:1561–74.
Riederer BM, Leuba G, Vernay A, Riederer IM. The role of the ubiquitin proteasome system in Alzheimer’s disease. Exp Biol Med (Maywood). 2011;236:268–76.
Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–35.
Gabbouj S, Ryhanen S, Marttinen M, Wittrahm R, Takalo M, Kemppainen S, Martiskainen H, Tanila H, Haapasalo A, Hiltunen M, Natunen T. Altered insulin signaling in Alzheimer’s disease brain—special emphasis on PI3K-Akt pathway. Front Neurosci. 2019;13:629.
Kang EB, Cho JY. Effect of treadmill exercise on PI3K/AKT/mTOR, autophagy, and Tau hyperphosphorylation in the cerebral cortex of NSE/htau23 transgenic mice. J Exerc Nutrition Biochem. 2015;19:199–209.
Zhang J, Guo Y, Wang Y, Song L, Zhang R, Du Y. Long-term treadmill exercise attenuates Abeta burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer’s disease. Neurosci Lett. 2018;666:70–7.
He XL, Yan N, Chen XS, Qi YW, Yan Y, Cai Z. Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol Rep. 2016;68:975–82.
Zhang M, Deng Y, Luo Y, Zhang S, Zou H, Cai F, Wada K, Song W. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem. 2012;120:1129–38.
Zhang M, Cai F, Zhang S, Zhang S, Song W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer’s progression in vivo. Sci Rep. 2014;4:7298.
Cheng H, **a B, Su C, Chen K, Chen X, Chen P, Zou Y, Yang X. PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure. J Trace Elem Med Biol. 2018;50:332–8.
Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–14.
Lee S, Choi BR, Kim J, LaFerla FM, Park JHY, Han JS, Lee KW, Kim J. Sulforaphane upregulates the heat shock protein co-chaperone CHIP and clears amyloid-beta and tau in a mouse model of Alzheimer’s disease. Mol Nutr Food Res. 2018;62: e1800240.
Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004;279:4869–76.
Acknowledgements
The authors gratefully acknowledge the financial support of the BWS21J001.
Funding
This work was supported by the BWS21J001.
Author information
Authors and Affiliations
Contributions
LFX, MZL, and ALW conceived and designed the study. LFX, MZL, ALW, and RL carried out the experiments. YJZ, YXC, and CL analyzed and interpreted the data. LFX, MZL, ALW, and ZXL wrote the first draft of the paper. KW and THW critically reviewed and edited the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental procedures were approved by the Laboratory Animal Welfare Ethics Committee of the Institute of Environmental and Operational Medicine, animal ethics review number IACUC of AMMS -04-2021-007.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
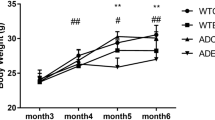
Treadmill Exercise Reduces Body Weight in APP/PS1 Transgenic Mice.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, L., Li, M., Wei, A. et al. Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid β and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J Neuroinflammation 19, 243 (2022). https://doi.org/10.1186/s12974-022-02607-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-022-02607-7