Abstract
Background
Pulmonary arterial hypertension (PAH) is a highly prevalent cardiopulmonary disorder characterized by vascular remodeling and increased resistance in pulmonary artery. Mitochondrial coiled–coil–helix–coiled–coil–helix domain (CHCHD)-containing proteins have various important pathophysiological roles. However, the functional roles of CHCHD proteins in hypoxic PAH is still ambiguous. Here, we aimed to investigate the role of CHCHD4 in hypoxic PAH and provide new insight into the mechanism driving the development of PAH.
Methods
Serotype 1 adeno‐associated viral vector (AAV) carrying Chchd4 was intratracheally injected to overexpress CHCHD4 in Sprague Dawley (SD) rats. The Normoxia groups of animals were housed at 21% O2. Hypoxia groups were housed at 10% O2, for 8 h/day for 4 consecutive weeks. Hemodynamic and histological characteristics are investigated in PAH. Primary pulmonary artery smooth muscle cells of rats (PASMCs) are used to assess how CHCHD4 affects proliferation and migration.
Results
We found CHCHD4 was significantly downregulated among CHCHD proteins in hypoxic PASMCs and lung tissues from hypoxic PAH rats. AAV1-induced CHCHD4 elevation conspicuously alleviates vascular remodeling and pulmonary artery resistance, and orchestrates mitochondrial oxidative phosphorylation in PASMCs. Moreover, we found overexpression of CHCHD4 impeded proliferation and migration of PASMCs. Mechanistically, through lung tissues bulk RNA-sequencing (RNA-seq), we further identified CHCHD4 modulated mitochondrial dynamics by directly interacting with SAM50, a barrel protein on mitochondrial outer membrane surface. Furthermore, knockdown of SAM50 reversed the biological effects of CHCHD4 overexpression in isolated PASMCs.
Conclusions
Collectively, our data demonstrated that CHCHD4 elevation orchestrates mitochondrial oxidative phosphorylation and antagonizes aberrant PASMC cell growth and migration, thereby disturbing hypoxic PAH, which could serve as a promising therapeutic target for PAH treatment.
Graphical Abstract

Similar content being viewed by others
Introduction
As a highly prevalent cardiopulmonary disorder, pulmonary arterial hypertension (PAH) is characterized with diffused vascular remodeling and pulmonary artery resistance, resulting in subsequent right ventricular dysfunction [1, 2]. Dysfunctional pulmonary arterial smooth muscle cells were manifested with abnormal proliferation and migration of PASMCs, together driving progressive remodeling of peripheral pulmonary arteries and augmented right ventricular afterload [3, 4]. However, the cellular and molecular mechanism modulating hypoxic PAH remains undefined. In addition, Metabolic reprogramming serves as a crucial driver in PAH, including hyper-glycolytic reprogramming, aberrant iron handling, insulin insensitivity, and defective sphingosine metabolism [5,6,7,8], however, whether and how mitochondrial dynamics regulates hypoxic PAH remain incompletely understood.
The coiled–coil–helix–coiled–coil–helix domain (CHCHD)-containing proteins are identified as a kind of conserved nucleus-encoded small mitochondrial proteins [9], among which have various important pathophysiological roles. Recently, CHCHD2 has been suggested to be associated with motor dysfunction and pathogenesis of Parkinson's disease (PD) [10, 11], particularly in mitochondrial homeostasis [12, 13]. Specifically, hepatocyte CHCHD2 contributed to liver fibrosis in nonalcoholic fatty liver disease (NAFLD) [14], whereas CHCHD3 was found to improve mitochondrial function via increasing mitochondrial ROS and promoting ferroptosis, by which modulated tumorigenesis [15]. In line with CHCHD2, CHCHD10 has been verified to be involved in multiple neurological alterations including Parkinson’s disease (PD), frontotemporal dementia (FTD), as well as Alzheimer's disease (AD) [13, 16]. CHCHD10 also serves as a mitochondrial modulator in adipose browning [42]. It plays a vital role in mitochondrial intermembrane space bridging and biogenesis of respiratory complexes [43]. In addition, SAM50 has been reported to be directly interacted with CHCHD3 and CHCHD6 [35, 44], by which SAM50 signaling enhanced OPA1 and Mfn1/2 expression while downregulating p-Drp1 and Fis1. This results in the formation of tight and parallel cristae, augmented expression of cardiac mitochondrial complex subunits, increased ATP generation, but reduced secretion of cytochrome C from mitochondria and oxidative damage [44]. Disruption of SAM50 and CHCHD3 crosstalk triggered mitochondrial cristae remodeling and resulted in palmitate-induced cardiomyocyte hypertrophy [45]. Herein, we found that CHCHD4 directly bound to SAM50 in PASMCs to regulate mitochondrial oxidative phosphorylation and aberrant proliferation and migration of PASMCs in hypoxic PAH.
Abnormal mitochondrial dynamics, including fission, fusion, and autophagy, are a key pathological feature of PAH. These alterations contribute to unrestricted cell proliferation and apoptosis resistance, leading to the development of PAH. Downregulation of Mfn2 impairs mitochondrial fusion, while excessive activation of Drp1 triggers aberrant mitochondrial fission, both of which are elevated in PAH patients [46,47,48,49,50]. Dysfunctional mitochondrial autophagy has been reported to generate excessive amount of reactive oxygen species (ROS), and it is also involved in modulating PAH by regulating AMPK/mTOR signal pathway [51, 52]. In current study, we found that CHCHD4 overexpression contributed to Mfn1 and Opa1 expression, and suppressed hypoxia induced Drp1 upregulation. We revealed that CHCHD4 modulated mitochondrial dynamics by directly interacting with SAM50, therefore targeting defective mitochondrial dynamics might serve as a potential approach for PAH treatment.
Limitations
Although we uncovered that CHCHD4 significantly restored mitochondrial oxidative phosphorylation in PASMCs, the link between defective mitochondria and proliferation and migration of PASMCs deserved further research. Moreover, we provide a new insight for CHCHD4-mediated protective effects in PAH, however, whether and how other CHCHDs regulates PAH progression is poorly understood. Here, male adult rats are utilized in our exploration, and the deviation attributed to sex difference should not be ignored. Importantly, insufficient evidence of CHCHD4 agonist hinders the clinical and translational value in this research.
Conclusion
We revealed that CHCHD4 was significantly downregulated among CHCHD proteins in rat PASMCs during hypoxia and lung tissues of rats with hypoxia-induced PAH. Interestingly, AAV serotype 1-induced CHCHD4 elevation conspicuously alleviates pulmonary artery resistance and vascular remodeling, and orchestrates mitochondrial oxidative phosphorylation in PASMCs. We also found overexpression of CHCHD4 impeded proliferation and migration of PASMCs. Mechanistically, through lung tissues bulk RNA-sequencing (RNA-seq), we further identified CHCHD4 modulated mitochondrial dynamics by directly interacting with SAM50, a barrel protein on mitochondrial outer membrane surface. Furthermore, knockdown of SAM50 reversed the biological effects of CHCHD4 overexpression in isolated PASMCs. We demonstrated that elevated CHCHD4 orchestrates mitochondrial oxidative phosphorylation and antagonizes aberrant proliferation and migration of PASMCs, thereby disturbing hypoxic PAH.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AAV1:
-
Serotype 1 adeno‐associated viral vector
- α-SMA:
-
Alpha smooth muscle Actin
- CHCHD4:
-
Coiled–coil–helix–coiled–coil–helix domain-containing protein 4
- CTR:
-
Control
- OE:
-
Overexpression
- OXPHOS:
-
Oxidative phosphorylation
- PAH:
-
Pulmonary arterial hypertension
- PASMCs:
-
Pulmonary artery smooth muscle cells of rats
- PCNA:
-
Proliferating cell nuclear antigen
- RVSP:
-
Right ventricular systolic pressure
References
Singh N, Dorfmuller P, Shlobin OA, Ventetuolo CE. Group 3 pulmonary hypertension: from bench to bedside. Circ Res. 2022;130:1404–22.
Naeije R, Richter MJ, Rubin LJ. The physiological basis of pulmonary arterial hypertension. Eur Respir J. 2022;59:2102334.
Zheng Y, et al. Deficiency of filamin A in smooth muscle cells protects against hypoxia-mediated pulmonary hypertension in mice. Int J Mol Med. 2023;51:1.
Chu C, et al. Swietenine alleviates vascular remodeling by enhancing mitophagy of pulmonary arterial smooth muscle cells in experimental pulmonary hypertension. Can J Cardiol. 2023. https://doi.org/10.1016/j.cjca.2023.01.003.
Cuthbertson I, Morrell NW, Caruso P. BMPR2 mutation and metabolic reprogramming in pulmonary arterial hypertension. Circ Res. 2023;132:109–26.
Wertheim BM, et al. Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension. JCI Insight. 2023. https://doi.org/10.1172/jci.insight.163932.
Chen J, et al. Sphingosine kinase 1 deficiency in smooth muscle cells protects against hypoxia-mediated pulmonary hypertension via YAP1 signaling. Int J Mol Sci. 2022;23:14516.
Gluschke H, et al. Autoimmunity to sphingosine-1-phosphate-receptors in systemic sclerosis and pulmonary arterial hypertension. Front Immunol. 2022;13: 935787.
Zhou ZD, Saw WT, Tan EK. Mitochondrial CHCHD-containing proteins: physiologic functions and link with neurodegenerative diseases. Mol Neurobiol. 2017;54:5534–46.
Fan L, et al. CHCHD2 p.Thr61Ile knock-in mice exhibit motor defects and neuropathological features of Parkinson’s disease. Brain Pathol. 2022;33: e13124.
Lu L, et al. CHCHD2 maintains mitochondrial contact site and cristae organizing system stability and protects against mitochondrial dysfunction in an experimental model of Parkinson’s disease. Chin Med J (Engl). 2022;135:1588–96.
Liu W, et al. Chchd2 regulates mitochondrial morphology by modulating the levels of Opa1. Cell Death Differ. 2020;27:2014–29.
Zhou W, Ma D, Tan EK. Mitochondrial CHCHD2 and CHCHD10: roles in neurological diseases and therapeutic implications. Neuroscientist. 2020;26:170–84.
Li Y, et al. Increased CHCHD2 expression promotes liver fibrosis in nonalcoholic steatohepatitis via Notch/osteopontin signaling. JCI Insight. 2022. https://doi.org/10.1172/jci.insight.162402.
Xue X, et al. Tumour cells are sensitised to ferroptosis via RB1CC1-mediated transcriptional reprogramming. Clin Transl Med. 2022;12: e747.
Jiang T, Wang Y, Wang X, Xu J. CHCHD2 and CHCHD10: Future therapeutic targets in cognitive disorder and motor neuron disorder. Front Neurosci. 2022;16: 988265.
**a W, et al. Chchd10 is dispensable for myogenesis but critical for adipose browning. Cell Regen. 2022;11:14.
Ding M, et al. CHCHD10 modulates thermogenesis of adipocytes by regulating lipolysis. Diabetes. 2022;71:1862–79.
Petrungaro C, et al. The Ca(2+)-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab. 2015;22:721–33.
Al-Habib H, Ashcroft M. CHCHD4 (MIA40) and the mitochondrial disulfide relay system. Biochem Soc Trans. 2021;49:17–27.
Thomas LW, et al. CHCHD4 regulates tumour proliferation and EMT-related phenotypes, through respiratory chain-mediated metabolism. Cancer Metab. 2019;7:7.
Thomas LW, et al. CHCHD4 confers metabolic vulnerabilities to tumour cells through its control of the mitochondrial respiratory chain. Cancer Metab. 2019;7:2.
Wang T, et al. C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab. 2021;33:531-546 e539.
Yang J, et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. J Clin Invest. 2012;122:600–11.
Whitley BN, Engelhart EA, Hoppins S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion. 2019;49:269–83.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–17.
Hong X, et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell. 2022;29:1298-1314 e1210.
Kleele T, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–9.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–59.
**e S, et al. Long-term activation of glucagon-like peptide-1 receptor by dulaglutide prevents diabetic heart failure and metabolic remodeling in type 2 diabetes. J Am Heart Assoc. 2022;11: e026728.
Mura M, Cecchini MJ, Joseph M, Granton JT. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology. 2019;24:1104–10.
Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–24.
Li X, et al. Mitochondria shed their outer membrane in response to infection-induced stress. Science. 2022;375:eabi4343.
Takeda H, et al. Mitochondrial sorting and assembly machinery operates by beta-barrel switching. Nature. 2021;590:163–9.
Ding C, et al. Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure. Sci Rep. 2015;5:16064.
Anderson JJ, Lau EM. Pulmonary hypertension definition, classification, and epidemiology in Asia. JACC Asia. 2022;2:538–46.
Hoeper MM, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–80.
Liu T, et al. Modulation of synaptic plasticity, motor unit physiology, and TDP-43 pathology by CHCHD10. Acta Neuropathol Commun. 2022;10:95.
Zhou Y, et al. USF1-CHCHD4 axis promotes lung adenocarcinoma progression partially via activating the MYC pathway. Discov Oncol. 2022;13:136.
Dickson-Murray E, Nedara K, Modjtahedi N, Tokatlidis K. The Mia40/CHCHD4 oxidative folding system: redox regulation and signaling in the mitochondrial intermembrane space. Antioxidants (Basel). 2021;10:592.
Thomas LW, Staples O, Turmaine M, Ashcroft M. CHCHD4 regulates intracellular oxygenation and perinuclear distribution of mitochondria. Front Oncol. 2017;7:71.
Lionello S, Marzaro G, Martinvalet D. SAM50, a side door to the mitochondria: the case of cytotoxic proteases. Pharmacol Res. 2020;160: 105196.
Ott C, et al. Sam50 functions in mitochondrial intermembrane space bridging and biogenesis of respiratory complexes. Mol Cell Biol. 2012;32:1173–88.
Xue RQ, et al. Pyridostigmine alleviates cardiac dysfunction via improving mitochondrial cristae shape in a mouse model of metabolic syndrome. Free Radic Biol Med. 2019;134:119–32.
Xue RQ, et al. Regulation of mitochondrial cristae remodelling by acetylcholine alleviates palmitate-induced cardiomyocyte hypertrophy. Free Radic Biol Med. 2019;145:103–17.
**ao F, Zhang R, Wang L. Inhibitors of mitochondrial dynamics mediated by dynamin-related protein 1 in pulmonary arterial hypertension. Front Cell Dev Biol. 2022;10: 913904.
Dasgupta A, et al. PINK1-induced phosphorylation of mitofusin 2 at serine 442 causes its proteasomal degradation and promotes cell proliferation in lung cancer and pulmonary arterial hypertension. FASEB J. 2021;35: e21771.
Ryan JJ, et al. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:865–78.
Feng W, et al. ERK/Drp1-dependent mitochondrial fission contributes to HMGB1-induced autophagy in pulmonary arterial hypertension. Cell Prolif. 2021;54: e13048.
Tian L, et al. Increased Drp1-mediated mitochondrial fission promotes proliferation and collagen production by right ventricular fibroblasts in experimental pulmonary arterial hypertension. Front Physiol. 2018;9:828.
Ornatowski W, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36: 101679.
Wei R, et al. Silencing TUFM inhibits development of monocrotaline-induced pulmonary hypertension by regulating mitochondrial autophagy via AMPK/mTOR signal pathway. Oxid Med Cell Longev. 2022;2022:4931611.
Acknowledgements
The authors would like to thank all the teachers and students who helped us.
Funding
None.
Author information
Authors and Affiliations
Contributions
XS and YW designed the studies, conducted experiments and drafted the manuscript. YW, GC and ZZ were responsible for data analyses and figure preparation. ZZ and YW contributed to conduct of experiments and manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal study was supported by the Animal Care and Use Committee of the Changhai Hospital of Navy Medical University (No.2021-0617).
Consent for publication
All authors consent to the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests with the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
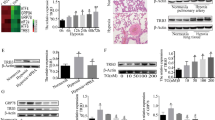
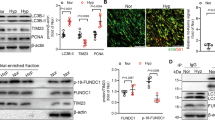
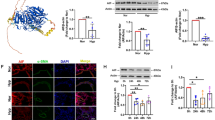
CHCHD4 is identified as a regulator of PAH. Related to Fig. 1. A Quantification of ratio of pulmonary arterial medial thickness to total vessel size (media/CSA) in indicated group (n = 6). Related to Fig. 1D. B Quantification of immunoblotting of CHCHD4 (n = 6). Related to Fig. 1C. C Quantitative analysis of α-SMA and CHCHD4 fluorescence intensity by Image J software (n=6). Related to Fig. 1E. D Representative immunofluorescence of Vimentin (green), CHCHD4 (red) and DAPI (blue) in lung tissues from indicated group. And quantitative analysis of CHCHD4 fluorescence intensity by Image J software (n = 6). Data are shown as the mean ± SEM. P value is showed in each figure. Figure S2. Protocols. A Experimental schedule of SD rats received AAV1-Chchd4 or CTR injection. Representative immunoblotting of CHCHD4 in lung tissues from animals with AAV1-CTR or Chchd4. Related to Fig. 3. B VSMCs, ECs and other cells were isolated from SD rats. Related to Fig. 1. C Experimental schedule of primary PASMCs received Chchd4 overexpression or knockdown in vitro. Related to Fig. 4. Figure S3. CHCHD4 affects hypoxia-induced migration in PASMCs. A The percent of wound closure of PASMCs received len-CTR or len-CHCHD4 transduction was counted (n = 6 fields from 3 independent experiments). Related to Fig. 4B. B The percent of wound closure of PASMCs received siCTR or siCHCHD4 transduction was counted (n = 6 fields from 3 independent experiments). Related to Fig. 4G. Data are shown as the mean ± SEM. P value is showed in each figure. Figure S4. Overexpression of CHCHD4 improves hypoxia-induced mitochondrial dysfunction. Related to Fig. 5. A Quantification of basal respiration, ATP production–coupled respiration, maximal respiration, and spare respiratory capacity from oxygen consumption rate (OCR) in PASMCs. Related to Fig. 5G. B Quantification of glycolysis, glycolytic capacity and glycolytic reserve from extracellular acidification rate (ECAR) in PASMCs. Related to Fig. 5H. Data are shown as the mean ± SEM. P value is showed in each figure. Figure S5. CHCHD4 modulates mitochondrial dynamics. Related to Fig. 6. A. Quantification of immunoblotting of Opa1, Drp1 and Mfn1 in lung PA tissues from indicated groups (n = 6). Related to Fig. 6C. B Quantification of immunoblotting of Opa1, Drp1 and Mfn1 in isolated PASMCs from indicated groups (n = 6). Related to Fig. 6D. Data are shown as the mean ± SEM. P value is showed in each figure. Figure S6. SAM50 knockdown abolishes the protective effects of CHHCD4 during hypoxia. Related to Fig. 7. Quantification of immunoblotting of CHCHD4, SAM50, Opa1, Drp1 and Mfn1 in isolated PASMCs from indicated groups (n = 6). Related to Fig. 7C.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Zeng, Z., Zeng, Z. et al. Elevated CHCHD4 orchestrates mitochondrial oxidative phosphorylation to disturb hypoxic pulmonary hypertension. J Transl Med 21, 464 (2023). https://doi.org/10.1186/s12967-023-04268-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04268-3