Abstract
Background
Atherosclerosis is a chronic vascular disease posing a great threat to public health. We investigated whether rosuvastatin (RVS) enhanced autophagic activities to inhibit lipid accumulation and polarization conversion of macrophages and then attenuate atherosclerotic lesions.
Methods
All male Apolipoprotein E-deficient (ApoE−/−) mice were fed high-fat diet supplemented with RVS (10 mg/kg/day) or the same volume of normal saline gavage for 20 weeks. The burden of plaques in mice were determined by histopathological staining. Biochemical kits were used to examine the levels of lipid profiles and inflammatory cytokines. The potential mechanisms by which RVS mediated atherosclerosis were explored by western blot, real-time PCR assay, and immunofluorescence staining in mice and RAW264.7 macrophages.
Results
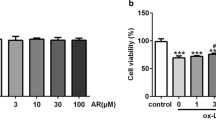
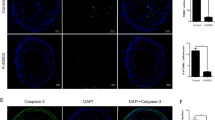
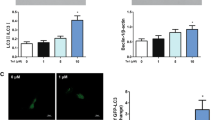
Our data showed that RVS treatment reduced plaque areas in the aorta inner surface and the aortic sinus of ApoE−/− mice with high-fat diet. RVS markedly improved lipid profiles and reduced contents of inflammatory cytokines in the circulation. Then, results of Western blot showed that RVS increased the ratio LC3II/I and level of Beclin 1 and decreased the expression of p62 in aortic tissues, which might be attributed to suppression of PI3K/Akt/mTOR pathway, hinting that autophagy cascades were activated by RVS. Moreover, RVS raised the contents of ABCA1, ABCG1, Arg-1, CD206 and reduced iNOS expression of arterial wall, indicating that RVS promoted cholesterol efflux and M2 macrophage polarization. Similarly, we observed that RVS decreased lipids contents and inflammatory factors expressions in RAW264.7 cells stimulated by ox-LDL, accompanied by levels elevation of ABCA1, ABCG1, Arg-1, CD206 and content reduction of iNOS. These anti-atherosclerotic effects of RVS were abolished by 3-methyladenine intervention. Moreover, RVS could reverse the impaired autophagy flux in macrophages insulted by chloroquine. We further found that PI3K inhibitor LY294002 enhanced and agonist 740 Y-P weakened the autophagy-promoting roles of RVS, respectively.
Conclusions
Our study indicated that RVS exhibits atheroprotective effects involving regulation lipid accumulation and polarization conversion by improving autophagy initiation and development via suppressing PI3K/Akt/mTOR axis and enhancing autophagic flux in macrophages.
Similar content being viewed by others
Background
Atherosclerosis is characterized by ascensive buildup of plaque in the arterial wall with perturbation of lipid metabolism and vascular inflammation [1]. Endothelium dysfunction results in subendothelial accumulation of oxidized low-density lipoprotein (ox-LDL), and transmigration of monocytes into arterial wall where they differentiate into macrophages that engulf excessive ox-LDL to generate lipid-laden foam cells, which ultimately triggers the initiation and development of atherosclerosis [1,2,3]. Besides, macrophages produce pro-inflammatory mediators under ox-LDL stimulation, which aggravates the atherosclerotic lesions [4, 5].
Macrophages, an immune cell population with high heterogeneity in phenotype and function, can alter own polarization state to adapt to complex external conditions [6,7,8,9]. When exposed to pro-inflammatory substances, resting macrophages turn to develop classical M1 phenotype capable of producing inflammatory factors including IL-6, IL-1β, TNF-α, and iNOS, leading to inflammation amplification and atherosclerosis development [10,11,12]. On the contrary, the alternatively activated M2 macrophages are generated in response to IL-4 or IL-13, which release anti-inflammatory factors such as IL-10, Arginase-1 and then possess anti-atherosclerotic ability [13, 14].
Autophagy, a pro-survival intracellular process, has been demonstrated to attenuate burgeoning plaques via suppressing foam cell formation and weakening inflammatory response [15, 16]. It is reported that pro-atherosclerotic factors such as ox-LDL could block autophagic cascades, whereas up-regulated autophagy pathway effectively blunts atherosclerosis progression in vitro and in vivo [17,18,19,20]. It has been suggested that autophagy displays important roles in M2 macrophage formation and there exists an association of macrophage polarization with autophagy signal transduction. Phosphoinositide3-kniase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway serves as a major regulator inhibiting the initiation of autophagic activities [21, 22]. Moreover, inhibition of PI3K/Akt/mTOR pathway has been shown to improve cholesterol efflux capacity of macrophage-derived foam cells and weaken atherosclerotic plaque inflammation by enhancing autophagy [21, 23]. mTOR inhibitor rapamycin has been indicated to play beneficial roles in alleviating development of plaque lesions [51]. Besides, autophagy was reported to modulate the polarization conversion of macrophage phenotype [52]. The disorder of autophagy process had been indicated to be associated with the impairment of lipid clearance and pro-inflammatory phenotype formation of macrophages [52]. Based on this, we hypothesized that RVS mediated the activation of autophagy to encumber lipid accumulation and facilitate M2 phenotypic polarization of macrophages. 3-MA and Rap were used as antagonist and agonist separately and our findings revealed that the beneficial effects of RVS on favoring autophagy implementation was abrogated by 3-MA in RAW264.7 cells insulted by ox-LDL, followed by invalidation of lipid accumulation amelioration and M2-like polarization formation induced by RVS, whereas Rap administration contributed to the positive roles of RVS in autophagy enhancement and subsequent improvement of lipid d and augmentation of generation of M2 phenotype. These observations suggested that RVS treatment exerts atheroprotective effects involving in reduction of foam cell formation and inflammatory phenotype switch via strengthening upstream autophagy processes.
Then we further investigated relevant mechanisms underlying RVS affected the autophagic activities. CQ, a kind of blocker targeting autophagy flux, was found to increase levels of LC3II/I ratio and p62 content [53], accompanied by deterioration of weakened autophagy in macrophages with ox-LDL intervention, while this effect was significantly alleviated by RVS treatment. PI3K/Akt/mTOR was a classic pathway regulating autophagy initiation and there was evidence revealing that the agents capable of enhancing autophagy via suppressing PI3K/Akt/mTOR pathway reduced the endothelial cell apoptosis induced by oxLDL [54]. Moreover, the inactivation of PI3K/Akt/mTOR pathway was clarified to mediate macrophage autophagy and stabilize the rupture-prone atherosclerotic plaques [21]. Our findings showed that RVS reduced the phosphorylated levels of PI3K, Akt and mTOR and elevated the activity of downstream target ULK1 in vivo and in vitro. Then, we discovered that PI3K agonist 740 Y-P abrogated the beneficial effects of RVS on impelling autophagy-related processes, while PI3K inhibitor LY294002 reinforced RVS-triggered autophagy-promoting effects in RAW264.7 cells stimulated by ox-LDL. These data suggested that RVS increased occurrence and development of autophagy via inhibiting signal transduction of PI3K/Akt/mTOR pathway and improving autophagic flux in macrophages under the lipid-laden condition (Fig. 7).
Conclusions
In conclusion, the present study indicated that RVS intervention potently inhibited the atherosclerotic plaque development in ApoE−/− mice induced by high-fat diet. Our results provided the evidence that RVS was able to enhance autophagy activities via prohibiting activation of PI3K/Akt/mTOR pathway and increasing autophagic flux, thus leading to the anti-atherosclerotic effects involving suppression of lipid droplets accumulation and facilitation of anti-inflammatory M2 phenotype polarization, which thereby provided novel aspects into the molecular mechanisms of RVS against atherosclerosis development.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- RVS:
-
Rosuvastatin
- ApoE− / − :
-
Apolipoprotein E-deficient
- ox-LDL:
-
Oxidized low-density lipoprotein
- mTOR:
-
Mammalian target of rapamycin
- HMG-CoA:
-
3-Hydroxyl-3-methylglutaryl coenzyme A
- HE:
-
Hematoxylin and eosin
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- HDL-C:
-
High density lipoprotein cholesterol
- ATCC:
-
American Type Culture Collection
- DMEM:
-
Dulbecco’s modified Eagle medium
- 3-MA:
-
3-Methyladenine
- Rap:
-
Rapamycin
- CQ:
-
Chloroquine
- ORO:
-
Oil Red O
- SD:
-
Standard deviation
- Atgs:
-
Autophagy-related genes
References
Gimbrone MA, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36.
Legein B, Temmerman L, Biessen EAL, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013;70:3847–69.
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–21.
Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–6.
Yan YY, Song DD, Wu JD, Wang JN. Long non-coding RNAs link oxidized low-density lipoprotein with the inflammatory response of macrophages in atherogenesis. Front Immunol. 2020;11:24.
Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, et al. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque—role of activated macrophages and inflammatory cytokines. Circulation. 2002;106:3024–30.
Bobryshev YV. Intracellular localization of oxidized low-density lipoproteins in atherosclerotic plaque cells revealed by electron microscopy combined with laser capture microdissection. J Histochem Cytochem. 2005;53:793–7.
Yang Y, Wang J, Guo S, Pourteymour S, Xu Q, Gong J, Huang Z, Shen Z, Diabakte K, Cao Z, et al. Non-lethal sonodynamic therapy facilitates the M1-to-M2 transition in advanced atherosclerotic plaques via activating the ROS-AMPK-mTORC1-autophagy pathway. Redox Biol. 2020;32:101501.
Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–67.
Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–23.
Hume DA. The many alternative faces of macrophage activation. Front Immunol. 2015;6:370.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Lee CG, Homer R, Zhou Z, Lanone Z, Wang XM, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen QS, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194:809–21.
Sierra-Filardi E, Vega MA, Sanchez-Mateos P, Corbi AL, Puig-Kroger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215:788–95.
Pi S, Mao L, Chen J, Shi H, Liu Y, Guo X, Li Y, Zhou L, He H, Yu C, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy. 2020. https://doi.org/10.1080/15548627.2020.1741202.
Li W, Sultana N, Siraj N, Ward LJ, Pawlik M, Levy E, Jovinge S, Bengtsson E, Yuan XM. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J Cell Mol Med. 2016;20:1664–72.
Sergin I, Razani B. Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis. Trends Endocrinol Metab. 2014;25:225–34.
Itabe H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: from atherosclerosis to periodontitis. J Clin Biochem Nutr. 2012;51:1–8.
Perrotta I, Aquila S. The role of oxidative stress and autophagy in atherosclerosis. Oxid Med Cell Longev. 2015. https://doi.org/10.1155/2015/130315.
Mollace V, Gliozzi M, Musolino V, Carresi C, Muscoli S, Mollace R, Tavernese A, Gratteri S, Palma E, Morabito C, et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int J Cardiol. 2015;184:152–8.
Zhai CG, Cheng J, Mujahid H, Wang HF, Kong J, Yin Y, Li JF, Zhang Y, Ji XP, Chen WQ. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS ONE. 2014;9:e90563.
Jiang YQ, Kou JY, Han XB, Li XS, Zhong ZY, Liu ZN, Zheng YH, Tian Y, Yang LM. ROS-dependent activation of autophagy through the PI3K/Akt/mTOR pathway is induced by hydroxysafflor yellow A-sonodynamic therapy in THP-1 macrophages. Oxid Med Cell Longev. 2017. https://doi.org/10.1155/2017/8519169.
Zhang BC. Luteolin attenuates foam cells formation and apoptosis in Ox-LDL-stimulated macrophages by enhancing autophagy. J Am Coll Cardiol. 2016;67:S18–S18.
**ao Q, Che X, Cai B, Tao Z, Zhang H, Shao Q, Pu J. Macrophage autophagy regulates mitochondria-mediated apoptosis and inhibits necrotic core formation in vulnerable plaques. J Cell Mol Med. 2020;24:260–75.
Gotto AM. Treating hypercholesterolemia: Looking forward. Clin Cardiol. 2003;26:I21–8.
Oesterle: pleiotropic effects of statins on the cardiovascular system (vol 120, pg 229, 2017). Circ Res. 2018; 123:E20.
May MB, Glode A. Novel Uses For Lipid-Lowering Agents. J Adv Pract Oncol. 2016;7:181–7.
Nicholls SJ, Raichlen JS, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Nissen SE. Intravascular ultrasound evaluation of the effect of rosuvastatin versus atorvastatin on progression of coronary atherosclerosis: design of the saturn study. Atheroscl Suppl. 2008;9:202–202.
Gliozzi M, Walker R, Muscoli S, Vitale C, Gratteri S, Carresi C, Musolino V, Russo V, Janda E, Ragusa S, et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int J Cardiol. 2013;170:140–5.
Kuroda K, Otake H, Shinohara M, Kuroda M, Tsuda S, Toba T, Nagano Y, Toh R, Ishida T, Shinke T, Hirata K. Effect of rosuvastatin and eicosapentaenoic acid on neoatherosclerosis: the LINK-IT Trial. Eurointervention. 2019;15:E1099.
Stein EA, Dann EJ, Wiegman A, Skovby F, Gaudet D, Sokal E, Charng MJ, Mohamed M, Luirink I, Raichlen JS, et al. Efficacy of rosuvastatin in children with homozygous familial hypercholesterolemia and association with underlying genetic mutations. J Am Coll Cardiol. 2017;70:1162–70.
Thondapu V, Kurihara O, Yonetsu T, Russo M, Kim HO, Lee H, Soeda T, Minami Y, Jang IK. Comparison of rosuvastatin versus atorvastatin for coronary plaque stabilization. Am J Cardiol. 2019;123:1565–71.
Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–96.
Na EJ, Nam HY, Park J, Chung MA, Woo HA, Kim HJ. PI3K-mTOR-S6K signaling mediates neuronal viability via collapsin response mediator protein-2 expression. Front Mol Neurosci. 2017;10:288.
He J, Zhang G, Pang Q, Yu C, **ong J, Zhu J, Chen F. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 2017;284:1324–37.
Cao H, Jia Q, Yan L, Chen C, **ng S, Shen D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int J Mol Sci. 2019;20:6093.
Hui B, Hou X, Liu R, Liu XH, Hu Z. Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. 2021;264:118721.
Ma Y, Huang ZY, Zhou ZL, He XY, Wang Y, Meng C, Huang G, Fang NY. A novel antioxidant Mito-Tempol inhibits ox-LDL-induced foam cell formation through restoration of autophagy flux. Free Radical Biol Med. 2018;129:463–72.
Sathiyakumar V, Kapoor K, Jones SR, Banach M, Martin SS, Toth PP. Novel therapeutic targets for managing dyslipidemia. Trends Pharmacol Sci. 2018;39:733–47.
Kosmas CE, Silverio D, Sourlas A, Montan PD, Guzman E, Garcia MJ. Anti-inflammatory therapy for cardiovascular disease. Ann Transl Med. 2019;7:147.
Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–66.
Maguire EM, Pearce SWA, **ao QZ. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vascul Pharmacol. 2019;112:54–71.
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–52.
Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, Takigami M, Kondo T, Atsumi T. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis. 2013;22:910–8.
Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–8.
Pardali E, Makowski LM, Leffers M, Borgscheiper A, Waltenberger J. BMP-2 induces human mononuclear cell chemotaxis and adhesion and modulates monocyte-to-macrophage differentiation. J Cell Mol Med. 2018;22:5429–38.
Zhao K, Xu XS, Meng X, Li YL, Li JF, Chen WQ. Autophagy of monocytes attenuates the vulnerability of coronary atherosclerotic plaques. Coron Artery Dis. 2013;24:651–6.
Shao BZ, Han BZ, Zeng YX, Su DF, Liu C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sin. 2016;37:150–6.
Martinet W, De Meyer GRY. Autophagy in atherosclerosis. Curr Atheroscl Rep. 2008;10:216–23.
Abderrazak A, Couchie D, Mahmood DFD, Elhage R, Vindis C, Laffargue M, Mateo V, Buchele B, Ayala MR, El Gaafary M, et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE(2).Ki mice fed a high-fat diet. Circulation. 2015;131:1061–70.
** P, Jun L, Zhaolin Z. FGF21 induces autophagy-mediated cholesterol efflux to inhibit atherogenesis via RACK1 up-regulation. J Cell Mol Med. 2020;24:4992–5006.
Chen W, Li X, Guo S, Song N, Wang J, Jia L, Zhu A. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. 2019;70:486–97.
Peng S, Xu LW, Che XY, **ao QQ, Pu J, Shao Q, He B. Atorvastatin inhibits inflammatory response, attenuates lipid deposition, and improves the stability of vulnerable atherosclerotic plaques by modulating autophagy. Front Pharmacol. 2018;9:438.
Che J, Liang B, Zhang Y, Wang Y, Tang J, Shi G. Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in human endothelial cells. Cardiovasc Pathol. 2017;31:57–62.
Acknowledgements
The authors would like to acknowledge the Experimental medical research Center of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, for its support in biochemical analysis and technical help.
Funding
This present work was supported by the National Natural Science Foundation of China (Grant Nos. 81873518 and 81270353).
Author information
Authors and Affiliations
Contributions
XMG, LL and LH conceived and designed the experiments. XXZ performed the experiments, analyzed the data and wrote the manuscript. YTQ, XNW, HL contributed to the animal experiments and interpretation of data. CL and WBR participated in the contributed to data acquisition and statistical analysis. XMG, LL and LH supervised the study and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study process was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology.
Consent of publication
Not applicable.
Competing interests
The authors declare no conflicts of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Qin, Y., Wan, X. et al. Rosuvastatin exerts anti-atherosclerotic effects by improving macrophage-related foam cell formation and polarization conversion via mediating autophagic activities. J Transl Med 19, 62 (2021). https://doi.org/10.1186/s12967-021-02727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-021-02727-3