Abstract
Background
Hepatocellular carcinoma (HCC) is still fatal even after surgical resection. The purpose of this study was to analyze the prognostic factors of 5-year survival rate and to establish a model to identify HCC patients with gain of surgery combined with chemotherapy.
Methods
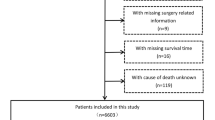
All patients with HCC after surgery from January 2010 to December 2015 were selected from the Surveillance, Epidemiology, and End Results (SEER) database. Univariate and multivariate logistic analysis were used to analyze the prognostic factors of patients, and the risk prediction model of 5-year survival rate of HCC patients was established by classical decision tree method. Propensity score matching was used to eliminate the confounding factors of whether to receive chemotherapy in high-risk group or low-risk group.
Results
One-thousand six-hundred twenty-five eligible HCC patients were included in the study. Marital status, α-fetoprotein (AFP), vascular infiltration, tumor size, number of lesions, and grade were independent prognostic factors affecting the 5-year survival rate of HCC patients. The area under the curve of the 5-year survival risk prediction model constructed from the above variables was 0.76, and the classification accuracy, precision, recall, and F1 scores were 0.752, 0.83, 0.842, and 0.836, respectively. High-risk patients classified according to the prediction model had better 5-year survival rate after chemotherapy, while there was no difference in 5-year survival rate between patients receiving chemotherapy and patients not receiving chemotherapy in the low-risk group.
Conclusions
The 5-year survival risk prediction model constructed in this study provides accurate survival prediction information. The high-risk patients determined according to the prediction model may benefit from the 5-year survival rate after combined chemotherapy.
Similar content being viewed by others
Introduction
Liver cancer ranks the fourth in the mortality of malignancy in the world, accounting for about 782,000 deaths each year, of which 85% are hepatocellular carcinoma (HCC) [1]. At present, surgical treatment is the most important curative treatment for patients with HCC, but the recurrence rate after 5 years is more than 50%, and the overall 5-year survival rate is only 18% [2, 3]. So, how can we reduce postoperative recurrence and improve postoperative survival in HCC patients? Recently, adjuvant therapy has been shown to improve survival in patients after HCC surgery. In a study of 200 patients with postoperative HCC, the researchers found that adjuvant transarterial chemoembolization significantly improved disease-free survival in patients with tumor size > 5 cm [4]. In a systematic review of 277 patients after HCC surgery, adjuvant immunotherapy was found to reduce the recurrence rate of the disease [5]. There were also some trials found that antiviral therapy could improve the prognosis of patients with HBV or HCV after HCC surgery [6, 1).
Determination of independent risk factors
Univariate (Fig. 2A) and multivariate (Fig. 2B) logistic analyses were conducted in the training group to obtain independent risk factors. Univariate analysis of the clinical parameters showed that marital status, grade, AFP level, vascular invasion, tumor size, number of lesions, and T stage were related to the 5-year CSD of patients. Multivariate analysis showed that marital status, grade, AFP, vascular invasion, tumor size, and number of lesions were independent risk factors for 5-year CSD of patients. We found that married was a good prognostic factor for HCC, and AFP-positive and vascular invasion suggested a poor prognosis. And the lower the degree of differentiation, the larger the tumor volume, and the more the number of tumors, the worse the prognosis.
Construction and verification of decision tree model
The independent risk factors derived from multivariate logistic analysis of the training group were used to construct a risk prediction model for 5-year CSD using a decision tree algorithm. The model constructed is shown in Figs. 3 and 4. Figure 3 shows the results of classifying patients without vascular invasion using the decision tree model. One-hundred seventy-one (16.3%, 171/1047) patients without vascular invasion were at high risk of CSD for 5 years. It can be observed from the figure that tumor size > 5cm is a risk factor for 5-year CSD (32.5%, 129/397), and patients with poorly and undifferentiated stage are high-risk groups for 5-year CSD (77.9%, 74/95). Figure 4 shows the results of classifying patients with vascular invasion using the decision tree model. One-hundred sixty-six (65.6%, 166/253) patients with vascular invasion were at high risk of CSD for 5 years. Consistent with the above results, tumor size > 5cm (55%, 44/80) and poorly and undifferentiated stage (93%, 93/100) are the main risk factors for CSD 5 years after liver cancer surgery. Then, we calculated the calibration curve of the model and found that the model had good fitting ability (Fig. 5A). We compared the ROC (Fig. 5B) of decision tree and logistic regression and found that the decision tree model (AUC = 0.76) had stronger prediction ability than logistic regression (AUC = 0.679). Then, we determined the threshold (threshold = 0.64) of the model according to the precision and recall (Fig. 5C). Patients were classified as high (survival rate ≤ 0.64) and low risk (survival rate > 0.64) according to this threshold. We also calculated the F1 (F1 = 0.836, Fig. 5D) and classification accuracy (classification accuracy = 0.752, Fig. 5E) of the model when the model threshold was 0.64. In the validation set, when the threshold was 0.64, AUC, classification accuracy, precision, recall, and F1 scores were 0.729, 0.757, 0.873, 0.824, and 0.848, respectively (Table 2). According to the model, all patients (n = 1625) with HCC undergoing surgery could be divided into two groups, of which 413 cases were high-risk group and 1212 cases were low-risk group (Additional file 1). These data suggested that the decision tree model had good prediction performance.
Effect of surgery combined with chemotherapy on high-risk and low-risk patients
To further explore the effect of surgery combined with chemotherapy on the prognosis of HCC patients, the high-risk group and low-risk group were further divided into two subgroups according to whether or not they had received chemotherapy. In the high-risk group, there was a significant difference in AFP between surgery alone and surgery combined with chemotherapy. In order to eliminate this confounding factor, we treated with PSM. After PSM correction, there was a significant difference in 5-year CSD between the two groups. The 5-year survival rate of patients treated with surgery alone was 15.5% (11/71), and that of patients treated with surgery combined with chemotherapy was 35.2% (25/71) (Table 3). In the low-risk group, there were significant differences in AFP, lesion, and grade between surgery alone and surgery combined with chemotherapy. We used PSM to eliminate these confounding factors. We found no difference in 5-year CSD between the two groups. These data suggested that surgery combined with chemotherapy can significantly improve the prognosis of HCC patients in the high-risk group, but it has no effect on the prognosis of HCC patients in the low-risk group (Table 4).
Discussion
The progress of surgical resection, ablation, and liver transplantation has improved the prognosis of HCC patients to some extent, but compared with other common human cancers, the long-term survival rate of HCC patients is still not ideal due to the high recurrence rate and lack of effective adjuvant therapy [20, 21]. Therefore, we must carry out hierarchical management and targeted treatment for postoperative patients with different risk levels in order to improve the long-term survival rate of patients with liver cancer. In this study, we found that tumor size, vascular invasion, AFP level, and number of lesions were independent risk factors for 5-year CSD through univariate and multivariate logistic regression analysis. Married was a good prognostic factor for HCC, and AFP-positive and vascular invasion suggested a poor prognosis. And the lower the degree of differentiation, the larger the tumor volume, and the more the number of tumors, the worse the prognosis. Previous studies have shown that tumor size, vascular invasion, AFP level, and number of lesions may affect the prognosis of patients with HCC, which is consistent with the results of this study [22,23,24]. Interestingly, in this study, it was found that marital status was also an independent risk factor for 5-year CSD. This is in kee** with previous reports that married patients had better 5-year HCC cause-specific survival than did unmarried patients (46.7% vs 37.8%) [25]. Marital status is an important prognostic factor for survival in patients with HCC treated with surgical resection.
There have also been previous reports on the postoperative prognosis model of HCC. Shim et al. established the survival nomogram of postoperative HCC patients (AUC = 0.66) [26]. This study also constructs a logistic regression model (AUC = 0.679). In contrast, the decision tree model (AUC = 0.760) in this study has better prediction performance. It seems to have greater clinical application potential. In the present study, vascular invasion, tumor size, and poor differentiation were the main risk factors for 5-year CSD in HCC patients after surgery, which is in kee** with previous studies [27, 28]. The prognosis of patients with vascular invasion, tumor size > 5cm, or poorly stage is poor. The decision tree prediction model in this study can accurately predict the high-risk group of patients with 5-year CSD after HCC surgery, help to realize patient-specific early diagnosis and treatment, and further improve the prognosis of HCC patients.
In recent years, some studies have found that surgical resection of HCC combined with chemotherapy can improve the postoperative survival rate [29,30,31]. However, there are no clinical guidelines recommending the routine use of surgery combined with chemotherapy for HCC patients because the beneficiaries are still uncertain. In this study, for the high-risk and low-risk patients divided based on the decision tree model, in the high-risk patients, the prognosis was significantly improved after surgery combined with chemotherapy, while in the low-risk patients, there was no significant change in CSD 5 years after surgery combined with chemotherapy. This means that the prognostic model established in this study can provide a reference for guiding the management of postoperative adjuvant chemotherapy.
The data source of this study is SEER database, which is an important resource for practical research in oncology. One-thousand six-hundred twenty-five HCC patients with complete clinical data were included. The characteristic distribution of the data is normal, and the model has good prediction performance in both training set and verification set, which provides a sufficient and reliable basis for further clinical application. However, this study also has some limitations. Because this study is based on a public database, the collection of clinical data is limited by the items provided in the data set, and it is impossible to explore more possible prognostic factors. In addition, the prognostic risk prediction model constructed in this study still needs external validation to further confirm its effectiveness.
Conclusions
The 5-year CSD prediction model based on decision tree algorithm provides accurate prediction information. The high-risk patients determined by the prediction model may benefit from the 5-year survival after surgery combined with chemotherapy. The prediction model is expected to provide reference for postoperative management of patients with HCC in the future.
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251–9.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Qi YP, Zhong JH, Liang ZY, Zhang J, Chen B, Chen CZ, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217(4):739–44.
Zhu GQ, Shi KQ, Yu HJ, He SY, Braddock M, Zhou MT, et al. Optimal adjuvant therapy for resected hepatocellular carcinoma: a systematic review with network meta-analysis. Oncotarget. 2015;6(20):18151–61.
Xu J, Li J, Chen J, Liu ZJ. Effect of adjuvant interferon therapy on hepatitis b/c virus-related hepatocellular carcinoma after curative therapy - meta-analysis. Adv Clin Exp Med. 2015;24(2):331–40.
**a BW, Zhang YC, Wang J, Ding FH, He XD. Efficacy of antiviral therapy with nucleotide/nucleoside analogs after curative treatment for patients with hepatitis B virus-related hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(4):458–68.
Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–403.
Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int. 2013;2013:310427.
Podgorelec V, Kokol P, Stiglic B, Rozman I. Decision trees: an overview and their use in medicine. J Med Syst. 2002;26(5):445–63.
Mitra AP, Skinner EC, Miranda G, Daneshmand S. A precystectomy decision model to predict pathological upstaging and oncological outcomes in clinical stage T2 bladder cancer. BJU Int. 2013;111(2):240–8.
Cao F, Shen L, Qi H, **e L, Song Z, Chen S, et al. Tree-based classification system incorporating the HVTT-PVTT score for personalized management of hepatocellular carcinoma patients with macroscopic vascular invasion. Aging (Albany NY). 2019;11(21):9544–55.
Wu Z, Chen W, Ouyang T, Liu H, Cao L. Management and survival for patients with stage-I hepatocellular carcinoma: an observational study based on SEER database. Medicine (Baltimore). 2020;99(41):e22118.
Zheng L, Zhang CH, Lin JY, Song CL, Qi XL, Luo M. Comparative effectiveness of radiofrequency ablation vs. surgical resection for patients with solitary hepatocellular carcinoma smaller than 5 cm. Front. Oncol. 2020;10:399.
Li W, **ao H, Wu H, Xu X, Zhang Y. Liver transplantation versus liver resection for stage I and II hepatocellular carcinoma: results of an instrumental variable analysis. Front Oncol. 2021;11:592835.
Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96(9):e5904.
Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2014;120(23):3707–16.
Godec P, Pancur M, Ilenic N, Copar A, Strazar M, Erjavec A, et al. Democratized image analytics by visual programming through integration of deep models and small-scale machine learning. Nat Commun. 2019;10(1):4551.
Stekhoven DJ, Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8.
Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181–200.
Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54(3):757–9.
Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–92.
Liu Y, Wang ZX, Cao Y, Zhang G, Chen WB, Jiang CP. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis Int. 2016;15(3):266–74.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–3.
Wu C, Chen P, Qian JJ, ** SJ, Yao J, Wang XD, et al. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: an analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget. 2016;7(48):79442–52.
Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–46.
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–43.
Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol. 2016;64(3):601–8.
Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309–16.
Choi JW, Park JY, Ahn SH, Yoon KT, Ko HK, Lee DY, et al. Efficacy and safety of transarterial chemoembolization in recurrent hepatocellular carcinoma after curative surgical resection. Am J Clin Oncol. 2009;32(6):564–9.
Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198(3):313–8.
Acknowledgements
We would like to thank the entire staff of the National Cancer Institute who participated in the Surveillance, Epidemiology, and End Results (SEER) project.
Funding
The project was funded by The Special Project of the First Affiliated Hospital, Chengdu Medical College [Grant No. CYFY2019ZD03], the School Foundation of Chengdu Medical College [Grant No. CYZYB21-05], and the Project of Chengdu Medical Research [Grant No. 2021015], and Science and Technology Department of Sichuan Province [Grant No. 2023NSFSC1249].
Author information
Authors and Affiliations
Contributions
KX, JW, WZ, conceptualization and methodology. JH and NG, wrote the main manuscript text. DL, YD, DL, and JC, software, data curation, visualization, and investigation. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable. SEER is a publically available anonymous data source, so this study was not reviewed by a Human Subjects Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Clinical Characteristics of 1625 eligible patients included in this study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, J., Gong, N., Li, D. et al. Identifying hepatocellular carcinoma patients with survival benefits from surgery combined with chemotherapy: based on machine learning model. World J Surg Onc 20, 377 (2022). https://doi.org/10.1186/s12957-022-02837-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02837-2