Abstract
Osteosarcoma (OS) derived small extracellular vesicles (OS-sEVs) have been shown to induce the formation of cancer-associated fibroblasts (CAFs), characterized by elevated pro-inflammatory factor expression and enhanced migratory and contractile abilities. These CAFs play a crucial role in priming lung metastasis by orchestrating the pre-metastatic niche (PMN) in the lung. Disrupting the communication between OS-sEVs and lung fibroblasts (LFs) emerges as a potent strategy to hinder OS pulmonary metastasis. Our previously established saponin-mediated cargo-elimination strategy effectively reduces the cancer-promoting ability of tumor-derived small extracellular vesicles (TsEVs) while preserving their inherent targeting capability. In this study, we observed that cargo-eliminated OS-sEVs (CE-sEVs) display minimal pro-tumoral and LFs activation potential, yet retain their ability to target LFs. The uptake of OS-sEVs by LFs can be concentration-dependently suppressed by CE-sEVs, preventing the conversion of LFs into CAFs and thus inhibiting PMN formation and pulmonary metastasis of OS. In summary, this study proposes a potential strategy to prevent LFs activation, PMN formation in the lung, and OS pulmonary metastasis through competitive inhibition of OS-sEVs’ function by CE-sEVs.
Similar content being viewed by others
Introduction
Osteosarcoma (OS) is the most common malignant tumor of bone [1, 2]. Since the introduction of systematic chemotherapy, the 5-year survival rate of non-metastatic OS patients has been increased from 20% to over 60%. Pulmonary metastasis is the most troublesome situation in OS patients with an about 16.5% occurrence rate [1, 3]. By contrast, the 5-year survival rate dramatically drops below 20% when pulmonary metastasis occurs [1, 3]. Furthermore, its detection is still a great challenge, which usually results in a delayed treatment. Thus, preventing pulmonary metastasis is a major issue in OS treatment to effectively reduce the mortality. However, up to now, no specific intervention method has been successfully practiced in clinics.
In the past two decades, a pre-metastasis niche (PMN)-based mechanism has been revealed to explain the organotropism of cancer cell migration in metastasis [4]. The PMN is defined as the remolded microenvironment that is favorable for the colonization and outgrowth of tumor cells in specific distant organs [5]. The PMN formation is tightly associated with the interplay between primary tumor cells’ secretomes and the microenvironment in specific organs. This theory has also been proved in pulmonary metastasis development of OS. For example, OS cell secreted glycoprotein ANGPTL2 can promote neutrophil recruitment, thereby perpetuating a chronic inflammation in lung [6]; OS cells-derived COL6A1 remodels the extracellular matrix of local lung microenvironment by promoting inflammatory cytokines and chemokines production [7]; The fusion protein Rab22a-NeoF1 derived from OS cells and its partner PYK2 cause the recruitment of bone marrow-derived macrophages to the lung and M2-type polarization of lung macrophages, and subsequently establish an immunosuppressive microenvironment in lung [8]. Thus, interfering the distant communication between the OS cells and lung microenvironment would have the potential to inhibit the PMN formation and further metastasis.
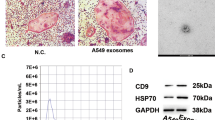
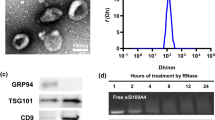
Small extracellular vesicles (sEVs) are crucial mediators in intercellular communications. These nano-sized (30 –150 nm) vesicles with lipid-bilayer membrane can load various cargos (mainly protein and RNA) of parent cells and effectively transport them to recipient cells and further induce specific responses [ In summary, by eliminating original cargos of OS-sEVs through saponin treatment, a non-tumor promoting and non-LFs activating subtype of OS-sEVs (CE-sEVs) is obtained. CE-sEVs possess similar targeting abilities for LFs as OS-sEVs, and can effectively competing and reducing the uptake of OS-sEVs by LFs. In vitro and in vivo experiments confirmed that the massive co-existed of CE-sEVs inhibits OS-sEVs-induced LFs activation and lung PMN formation through competitive cellular uptake strategies. Furthermore, in both experimental and spontaneous metastasis OS models in mice, CE-sEVs treatment reduced the lung metastasis of OS cells (Fig. 8). This study provides an intervention strategy for preventing LFs activation, pulmonary PMN formation, and OS lung metastasis through the competitive inhibition of OS-sEVs function by CE-sEVs. Characterization of OS-sEVs and CE-sEVs. (A) Western blot analysis of sEVs characteristic markers (CD9, CD63, and TSG101) and non-sEVs marker GM130 in MNNG cells and OS-sEV. (B) Representative TEM images of OS-sEVs and CE-sEVs. Scale bar: 100 nm. (C) Particle size distribution of OS-sEVs and CE-sEVs. (D) Quantification of the mean protein concentration per particle of OS-sEVs and CE-sEVs (n = 3). (E) Sliver staining image of total proteins in OS-sEVs (1 × 1010 particles) and CE-sEVs (1 × 1010 particles) and (F) the quantification of the relative protein content of OS-sEVs and CE-sEVs (n = 3). (G) RNA enrichment analysis depicted in FU per nt of total RNA contents in OS-sEVs (1 × 1010 particles) and CE-sEVs (1 × 1010 particles). (H) Total RNA contents in OS-sEVs (1 × 1010 particles) and CE-sEVs (1 × 1010 particles) by SYTO™ RNA staining (n = 3). *** P < 0.001; # P < 0.0001 CE-sEVs exhibit no tumor-promoting ability. (A) CCK-8 assay detects the proliferation of MNNG cells after treated with OS-sEVs and CE-sEVs, the results confirm that OS-sEVs promoted OS cell proliferation, while CE-sEVs did not have such effect (n = 3). (B) Transwell assay detects the migration of MNNG cells after treated with OS-sEVs and CE-sEVs, the results confirm that OS-sEVs promoted OS cell migration, while CE-sEVs did not have such effect (n = 3). (C) Tumor resection images after intravenous intervention with OS-sEVs (n = 5), CE-sEVs (n = 5), and control (n = 5) in the nude mouse subcutaneous tumor model, administered three times per week. (D) Quantitation of tumor weight (mean ± SD). (E) Quantitation of tumor volume (mean ± SD). ns P > 0.05; * P < 0.05; *** P < 0.001 CE-sEVs competitively suppressed the uptake of OS-sEVs by LFs. (A) Representative images of HFL-1 cells treated with DiO-labelled OS-sEVs or DiO-labelled CE-sEVs detected by flow cytometry. (B) Representative images of the HFL-1 cells uptake of DiO-labelled OS-sEVs (green) or DiO-labelled CE-sEVs (green), CE-sEVs show similar internalized ability to OS-sEVs. (C) IF images of OS-sEVs (red, DiR) and CE-sEVs (red, DiR) co-localization with LFs (green, S100A4). (D) IF show the uptake efficiency of HFL-1 towards OS-sEVs in different CE-sEVs concentration (0 particles/mL, 5 × 109 particles/mL, and 1 × 1010 particles/mL in group 2, group 3, and group 4, respectively). (E) Flow cytometry show the uptake efficiency of HFL-1 towards OS-sEVs in different CE-sEVs concentration (0 particles/mL, 5 × 109 particles/mL, and 1 × 1010 particles/mL in group 2, group 3, and group 4, respectively). (F) Representative ex vivo fluorescence images of main organs. Mice were intravenously injected with DiR-labeled OS-sEVs, followed by interventions with blank, PBS, and CE-sEVs, respectively. After 24 h, the main organs were harvested for ex vivo fluorescence observation. (G) Statistical analysis of the FI in lung (n = 3). ns P > 0.05; *** P < 0.001; # P < 0.0001 CE-sEVs exhibit no LFs-activating ability. (A) Western blot analysis the expression of TGF-β, a key mediator of LFs activation, in OS-sEVs and CE-sEVs (n = 3). (B) RT-qPCR analysis the expression of the genes associated with LFs activation in HFL-1 cells treated with OS-sEVs or CE-sEVs for 24 h. (C) Representative images of wound healing analysis of HFL-1 cells cultured with OS-sEVs or CE-sEVs for 0 h, 12 h, and 24 h. (D) quantification of the migration rate of HFL-1 cells cultured with OS-sEVs or CE-sEVs for 0 h, 12 h, and 24 h (n = 3). (E) Representative images of collagen matrix contraction analysis of HFL-1 cells cultured with OS-sEVs or CE-sEVs for 0 h, 24 h, and 72 h and (F) quantification of the contraction rate of HFL-1 cells cultured with OS-sEVs or CE-sEVs for 0 h, 24 h, and 72 h (n = 2). ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001; # P < 0.0001 LFs activation induced by OS-sEVs efficiently suppressed by CE-sEVs. (A) RT-qPCR analysis the expression of the genes associated with LFs activation in HFL-1 cells treated with OS-sEVs and OS-sEVs + CE-sEVs for 24 h (n = 3). (B) Representative images of wound healing analysis of HFL-1 cells cultured with OS-sEVs or OS-sEVs + CE-sEVs for 0 h, 12 h, and 24 h. (C) quantification of the migration rate of HFL-1 cells cultured with OS-sEVs or OS-sEVs + CE-sEVs for 0 h, 12 h, and 24 h (n = 3). (E) Representative images of collagen matrix contraction analysis of HFL-1 cells cultured with OS-sEVs or OS-sEVs + CE-sEVs for 0 h, 24 h, and 72 h and (F) quantification of the contraction rate of HFL-1 cells cultured with OS-sEVs or OS-sEVs + CE-sEVs for 0 h, 24 h, and 72 h (n = 2). ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001; # P < 0.0001 CE-sEVs mediated competitive cellular uptake suppressed LFs activation and PMN formation. (A) Schematic representation of the detection of activated LFs and PMN, the mice were assigned into three groups: the control group treated with 1 × 109 particles OS-sEVs, the PBS group treated with 1 × 109 particles OS-sEVs + PBS, and the CE-sEVs group treated with 1 × 109 particles OS-sEVs + 1 × 1010 particles CE-sEVs. Treatments were given every other day. On day 7, lung tissues were collected follow by IF detection. (B) Representative IF image of LFs activation markers (S100A4, α-SMA, and FAP), scale bar: 100 μm. (C) Quantification of FI for LFs activation markers (S100A4, α-SMA, and FAP) (n = 3). (D) Representative IF image of PMN markers (FN, MMP9, and LOX), scale bar: 100 μm. (E) Quantification of FI for PMN markers (FN, MMP9, and LOX) (n = 3). ns P > 0.05; * P < 0.05; *** P < 0.001; # P < 0.0001 CE-sEVs efficiently suppressed the pulmonary metastasis of OS. (A) Schematic representation of the experimental metastasis model of OS. The mice were divided into three groups and pretreated with blank (control group), OS-sEVs (OS-sEVs group), OS-sEVs + CE-sEVs (CE-sEVs group) every three days. Then, MNNG cells were intravenous injection on day 12, mice were euthanized on day 28, and lungs were excised for observation of metastasis using BLI. (B) Representative ex vivo BLI of the lungs in experimental metastasis model, and the pulmonary metastasis of MNNG cells were calculated based on the lung’s FI value. (C) Quantification of lung’s FI in experimental metastasis model (n = 4/5). (D) Schematic representation of the spontaneous metastasis model of OS. The mice received MNNG cells inoculation into the tibia on day 0, and then divided into three groups: the control group (administered with blank), the PBS group (administered with PBS), and the CE-sEVs group (administered with CE-sEVs). Thrice-weekly interventions were performed. At the end of week 4, the mice were euthanized, and lungs were excised for observation of metastasis using BLI. (E) Representative ex vivo BLI of the primary tumors and lungs in spontaneous metastasis model, and the pulmonary metastasis of MNNG cells were calculated based on the lung’s FI value. (F) Quantification of lung’s FI in spontaneous metastasis model by BLI (n = 4/5). (G) Kaplan-Meier analysis of survival time in mice with spontaneous OS metastasis model (n = 8). ns P > 0.05; * P < 0.05; ** P < 0.01 Schematic illustration of the mechanisms how CE-sEVs prevent the formation of PMN formation in lung induced by OS-sEVs. CE-sEVs inhibit the LFs activation by competitively blocking the uptake of OS-sEVs. This inhibition ultimately prevented the modification of the local pulmonary microenvironment, resulting in the suppression of PMN formation and pulmonary metastasis of OS.Conclusion
Data availability
No datasets were generated or analysed during the current study.
References
Ritter J, Bielack SS, Osteosarcoma. Ann Oncol. 2010;21:vii320–325. https://doi.org/10.1093/annonc/mdq276. Suppl 7.
Isakoff MS, Bielack SS, Meltzer P, Gorlick R, Osteosarcoma. Current treatment and a collaborative pathway to Success. J Clin Oncol. 2015;33:3029–35. https://doi.org/10.1200/JCO.2014.59.4895.
Huang X, et al. Risk and clinicopathological features of osteosarcoma metastasis to the lung: a population-based study. J Bone Oncol. 2019;16:100230. https://doi.org/10.1016/j.jbo.2019.100230.
Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. https://doi.org/10.1038/nature04186.
Kaplan RN, Rafii S, Lyden D. Preparing the soil: the premetastatic niche. Cancer Res. 2006;66:11089–93. https://doi.org/10.1158/0008-5472.Can-06-2407.
Charan M, et al. Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget. 2020;11:510–22. https://doi.org/10.18632/oncotarget.27433.
Zhang Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473–92. https://doi.org/10.7150/thno.51245.
Zhong L, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;6:59. https://doi.org/10.1038/s41392-020-00414-1.
Lu M, Shao W, **ng H, Huang Y. Extracellular vesicle-based nucleic acid delivery. Interdisciplinary Med. 2023;1:e20220007. https://doi.org/10.1002/INMD.20220007.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–59. https://doi.org/10.1038/s41565-021-00931-2.
Macklin R, et al. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget. 2016;7:43570–87. https://doi.org/10.18632/oncotarget.9781.
Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. https://doi.org/10.1038/nature15756.
Mazumdar A, et al. Osteosarcoma-Derived Extracellular vesicles induce lung fibroblast reprogramming. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21155451.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. https://doi.org/10.1038/s41573-018-0004-1.
Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86. https://doi.org/10.1038/s41568-019-0238-1.
Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated fibroblasts. Physiol Rev. 2021;101:147–76. https://doi.org/10.1152/physrev.00048.2019.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. https://doi.org/10.3402/jev.v3.24641.
Arya SB, Collie SP, Parent CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2024;34:90–108. https://doi.org/10.1016/j.tcb.2023.06.006.
Matsumoto A, et al. Role of phosphatidylserine-derived negative surface charges in the Recognition and Uptake of Intravenously injected B16BL6-Derived exosomes by macrophages. J Pharm Sci. 2017;106:168–75. https://doi.org/10.1016/j.xphs.2016.07.022.
Guo Y, et al. Eliminating the original cargos of glioblastoma cell-derived small extracellular vesicles for efficient drug delivery to glioblastoma with improved biosafety. Bioact Mater. 2022;16:204–17. https://doi.org/10.1016/j.bioactmat.2022.02.013.
Tian Y, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9:1697028. https://doi.org/10.1080/20013078.2019.1697028.
Gustafson D, et al. Circulating small extracellular vesicles mediate vascular hyperpermeability in diabetes. Diabetologia. 2024;67:1138–54. https://doi.org/10.1007/s00125-024-06120-9.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. https://doi.org/10.1038/nrc1877.
Ji YD, et al. BML-111 suppresses TGF-β1-induced lung fibroblast activation in vitro and decreases experimental pulmonary fibrosis in vivo. Int J Mol Med. 2018;42:3083–92. https://doi.org/10.3892/ijmm.2018.3914.
Chen Y, et al. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int J Pharm. 2022;623:121953. https://doi.org/10.1016/j.ijpharm.2022.121953.
Ji Q, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun. 2020;11:1211. https://doi.org/10.1038/s41467-020-14869-x.
Kong J, et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer. 2019;18:175. https://doi.org/10.1186/s12943-019-1101-4.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–81. https://doi.org/10.1016/j.ccell.2016.09.011.
Zhuo S, et al. A potential opportunity for intervention of pre-metastatic niche. Front Oncol. 2022;12:980620. https://doi.org/10.3389/fonc.2022.980620. Ferroptosis.
Gumberger P, Bjornsson B, Sandström P, Bojmar L, Zambirinis CP. The Liver Pre-metastatic Niche in Pancreatic Cancer: a potential opportunity for intervention. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14123028.
Zhao L, et al. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. https://doi.org/10.1016/j.jconrel.2019.12.005.
Kaczanowska S et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184, 2033–2052.e2021, https://doi.org/10.1016/j.cell.2021.02.048 (2021).
**a C, et al. Sponge-like nano-system suppresses tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche. Acta Biomater. 2023;158:708–24. https://doi.org/10.1016/j.actbio.2023.01.009.
Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–17. https://doi.org/10.1038/nrc.2017.6.
Ren B, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17. https://doi.org/10.1186/s12943-018-0858-1.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a Distance. Dev Cell. 2019;49:347–60. https://doi.org/10.1016/j.devcel.2019.04.011.
Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. https://doi.org/10.1038/nature10694.
Gong Z, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. 2022;55:1483–e15001489. https://doi.org/10.1016/j.immuni.2022.07.001.
Wang M, et al. Tumor-derived exosomes drive pre-metastatic niche formation in lung via modulating CCL1(+) fibroblast and CCR8(+) Treg cell interactions. Cancer Immunol Immunother. 2022;71:2717–30. https://doi.org/10.1007/s00262-022-03196-3.
Zhou Y, et al. Peptide nano-blanket impedes fibroblasts activation and subsequent formation of pre-metastatic niche. Nat Commun. 2022;13:2906. https://doi.org/10.1038/s41467-022-30634-8.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 81572239) and the Shanghai Sixth People’s Hospital Cultivation Project (Grant number 202107) and the Shanghai Municipal Health Commission (Grant number 201940276).
Author information
Authors and Affiliations
Contributions
Shanyi Lin and Longqiang Shu contribute equally to this article. Shanyi Lin: Conceptualization, Investigation, Writing – original draft. Longqiang Shu: Conceptualization, Investigation, Data curation. Yuhang Guo: Investigation. Ji Yuan: Investigation. Juntao Zhang: Investigation. Yunlong Yang: Conceptualization, Writing – review & editing, Funding acquisition. Ting Yuan: Conceptualization, Funding acquisition. Yang Wang: Conceptualization, Writing – review & editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Mice were procured from the Laboratory Animal Research Center of Shanghai Sixth People’s Hospital, with all procedures being sanctioned by the Animal Research Committee of Shanghai Sixth People’s Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, S., Shu, L., Guo, Y. et al. Cargo-eliminated osteosarcoma-derived small extracellular vesicles mediating competitive cellular uptake for inhibiting pulmonary metastasis of osteosarcoma. J Nanobiotechnol 22, 360 (2024). https://doi.org/10.1186/s12951-024-02636-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02636-9