Abstract
Mitochondria occupy a central role in the biology of most eukaryotic cells, functioning as the hub of oxidative metabolism where sugars, fats, and amino acids are ultimately oxidized to release energy. This crucial function fuels a variety of cellular activities. Disruption in mitochondrial metabolism is a common feature in many diseases, including cancer, neurodegenerative conditions and cardiovascular diseases. Targeting tumor cell mitochondrial metabolism with multifunctional nanosystems emerges as a promising strategy for enhancing therapeutic efficacy against cancer. This review comprehensively outlines the pathways of mitochondrial metabolism, emphasizing their critical roles in cellular energy production and metabolic regulation. The associations between aberrant mitochondrial metabolism and the initiation and progression of cancer are highlighted, illustrating how these metabolic disruptions contribute to oncogenesis and tumor sustainability. More importantly, innovative strategies employing nanomedicines to precisely target mitochondrial metabolic pathways in cancer therapy are fully explored. Furthermore, key challenges and future directions in this field are identified and discussed. Collectively, this review provides a comprehensive understanding of the current state and future potential of nanomedicine in targeting mitochondrial metabolism, offering insights for develo** more effective cancer therapies.
Similar content being viewed by others
Background
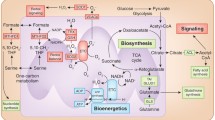
Mitochondria, the cell’s bioenergetic hubs, comprise four key components: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM), and the mitochondrial matrix (MM) [1]. They are central to cellular energy metabolism, playing a crucial role in the oxidation of various metabolic substrates, including carbohydrates, fats, and proteins. These substrates are converted into intermediates like acetyl coenzyme A (acetyl-CoA), which then enters the mitochondria for further processing in the tricarboxylic acid (TCA) cycle, underscoring the organelles’ critical role in energy production. Additionally, mitochondria are pivotal in regulating key cellular processes like redox balance, Ca2+ levels, apoptosis initiation, and biomacromolecule balance, thereby influencing cell survival and death [2].
Mitochondrial metabolic pathways predominantly encompass the TCA cycle, oxidative phosphorylation (OXPHOS), fatty acid oxidation (FAO) and glutamine (Gln) metabolism. These pathways are integral to the breakdown of biomacromolecules and the generation of energy [3]. Importantly, mitochondria serve as a primary source of reactive oxygen species (ROS), stemming from their involvement in various core metabolic processes. The production of ROS can disrupt the redox network, leading to the oxidation of lipids and proteins, mutations in mitochondrial DNA (mtDNA), and the induction of oxidative stress. These effects are implicated in the initiation and progression of a range of systemic diseases, including cancer, neurodegenerative disorders, diabetes, obesity, ischemic heart disease, hyperthyroidism, and phenylketonuria [4,5,42].
Innovative approaches have been proposed to address these challenges, notably through the integration of mitochondria-targeting molecules within nanoparticle frameworks. For instance, a mitochondria-targeted diketopyrrolopyrrole photosensitizer has been developed, enabling photodynamic and photothermal anti-cancer therapies by targeting mitochondria for heat and singlet oxygen generation [102]. Moreover, gold nanoparticles (AuNPs), enhanced with polymer and folate, accumulate in mitochondria, leading to oxidative stress and apoptosis. Notably, AuNPs disrupt glycolysis, reducing key enzymes through a c-Myc-dependent pathway, causing energy deprivation and inhibiting tumor growth, with minimal impact on non-tumor cells [103].
Inorganic nonmetallic materials
Inorganic nonmetallic nanodrugs have also demonstrated outstanding performance and broad application potential in treating tumors by inducing oxidative stress through targeting mitochondria. For example, iron-oxide magnetic nanoparticles conjugated with Dox effectively target mitochondrial dysfunction in breast cancer cells, inducing oxidative stress that leads to DNA damage, lipid peroxidation, and mitochondrial potential loss. This mitochondrial disruption halts the cell cycle and reduces cell migration, enhancing Dox delivery and increasing cancer cell mortality while potentially lowering toxicity to healthy cells, highlighting their promise in anticancer therapy [104]. Similarly, superparamagnetic iron oxide nanoparticles (SPIONs) demonstrate selective cytotoxicity in vitro against mitochondria isolated from oral cancer. Exposure to SPIONs increases ROS production, disrupts mitochondrial membrane potential, triggers cytochrome c release, and induces mitochondrial swelling. Furthermore, SPIONs decrease succinate dehydrogenase activity in cancerous mitochondria, suggesting their potential as therapeutic agents for oral cancer without significantly affecting non-cancerous cells [105]. Notably, CsI(Na)@MgO nanoparticles, paired with 5-aminolevulinic acid, present a streamlined radiodynamic therapy strategy enhancing tumor suppression. This combination targets mitochondria in cancer cells, increasing ROS production upon X-ray exposure and synergizing with DNA-targeted irradiation to intensify mitochondrial, DNA, and lipid damage [106]. Furthermore, ZnO nanoparticles effectively inhibit proliferation and induce apoptosis in human multiple myeloma cells, primarily through mitochondria-mediated pathways. Exposure to ZnO nanoparticles increases ROS production and decreases ATP levels, enhancing cytochrome C, APAF-1, caspase-9, and caspase-3 expression. This suggests a potent role for ZnO nanoparticles in triggering mitochondrial apoptosis, with minimal cytotoxic effects on peripheral blood mononuclear cells, highlighting their potential as therapeutic agents against multiple myeloma [107].
Organic nanoparticles
Organic nanocarriers offer significant advantages for drug delivery, including enhanced cellular penetration due to their small size and surface modification capabilities. These carriers exhibit high target specificity, improved drug stability, and controlled release mechanisms responsive to specific stimuli like pH or temperature. Additionally, their biocompatibility and low toxicity are crucial for reducing side effects and improving patient tolerance [108]. For instance, red-emissive carbon dots (RCDs) have been developed for PDT, with tunable ROS generation suitable for both aerobic and hypoxic conditions. These RCDs can produce both type I and type II ROS, owing to their specific core sizes and surface states. Their mitochondrial targeting ability enables them to activate cell death via mitochondrial pathways [109]. Interestingly, the nanocarrier HAL/3MA@X-MP, combining hexyl 5-aminolevulinate hydrochloride (HAL) and 3-methyladenine (3MA) within tumor cell-derived microparticles (X-MP), targets tumor cells. HAL induces sonosensitizer accumulation in mitochondria for effective ROS generation and mitochondrial damage, while 3MA inhibits mitophagy and downregulates PD-L1, enhancing immunogenic cell death and impeding immune checkpoint recognition [110]. Moreover, a mitochondria-targeted drug nanocarrier, prepared through host-guest interactions between α-cyclodextrin and polyethylene glycol (PEG), effectively combines a NO donor and a cinnamaldehyde prodrug for cancer treatment. This approach enhances oxidative stress by depleting GSH and generating peroxynitrite in mitochondria, leading to effective apoptosis in cancer cells and showing significant antitumor activity in hepatoma models [111].
Organic/inorganic hybrid nanoparticles
Organic/inorganic hybrid nanomaterials demonstrate significant advantages in drug delivery. By combining the stability and unique optical properties of inorganic components with the biocompatibility of organic components, these materials facilitate efficient drug loading and precise control over drug release, enhancing accumulation at targeted sites [112]. One commonly employed strategy is to integrate metals or metal compounds with other organic components to form composite nanomedicines. For instance, using bio-synthesized gold nanoclusters (Au NCs) paired with mitochondria-targeted aptamer-Pyro conjugates (ApPCs), this approach enhances uptake and mitochondrial targeting within cancer cells. When irradiated, it produces high levels of ROS, effectively killing cancer cells [139]. In addition, some novel bioactive nanosystems have been designed to achieve anti-tumor effects by modulating the mitochondrial metabolic pathways in cancer cells. A mitochondrial-targeting aggregation-induced emission luminogen (AIEgen), DCPy, is combined with living mitochondria to form a bioactive nanohybrid for deep-seated cancer treatment. This nanohybrid generates ROS under microwave irradiation, inducing apoptosis in cancer cells and reprogramming their metabolism from glycolysis to OXPHOS, thereby further enhancing the efficiency of microwave dynamic therapy [140]. Likewise, a novel cancer treatment strategy combines an aggregation-induced emission photosensitizer with bioactive mitochondria (Mito-AIEgen-lipid) to enhance PDT efficiency. This engineered living system shifts cell metabolism towards OXPHOS, inhibiting growth and triggering apoptosis [141].
Nanoparticles targeting mitochondria inhibit OXPHOS to enhance cancer treatment. (A) Organic nanoparticles, such as Bi-Ch, PEG-PCL, and IR-LND@Alb, inhibit OXPHOS and reverse hypoxia, thus enhancing the effectiveness of oxygen-sensitive therapies, including chemotherapy and PDT. (B) Organic/Inorganic hybrid nanoparticles, including PEG-GO@XN, PTX@GO-PEG-OSA, F-AgAps, and Au25(Capt)18, impede mitochondrial respiratory chain enzymes, resulting in increased ROS and reduced ATP production, leading to cell apoptosis and impairing mechanisms of cell migration and invasion while augmenting chemotherapy efficacy. (C) Other novel nanoparticles, such as BLG@TPGS, POPD@ATO@CPT-Py, ZIF-90@ATO@hemin@IRGD, and HM-NPs@G, also inhibit OXPHOS. Contrastingly, Mito-AIEgen-lipid shifts cellular metabolism towards OXPHOS, thereby inhibiting growth, triggering cell apoptosis, and enhancing the efficiency of PDT
Targeting ATP production
Various nanoparticles have been engineered to target ATP production in cancer cells (Fig. 5). For instance, the TPP-PPG@ICG nanocomposite, integrating a mitochondria-targeting ligand with ICG-loaded graphene, provides synergistic photodynamic and PTT activated by near-infrared light. It disrupts ATP synthesis and mitochondrial function in cancer cells, overcoming drug resistance and leading to cell death. Proven selective and effective in experiments, TPP-PPG@ICG shows promise as a safe and potent treatment for drug-resistant osteosarcoma [142]. In addition, the HNHA-GC nanocomposite disrupts cancer cell metabolism by blocking mitochondrial respiration and glycolysis, crucial for ATP production. It releases calcium, 10-hydroxy CPT, and glucose oxidase (GOD) at tumor sites, causing mitochondrial dysfunction and inhibiting glycolysis. This trigger increased ROS and acidity, enhancing calcium overload [143]. Interestingly, the LMGC nanoparticle is designed with a liquid metal core, surface-functionalized with GOD and coated with calcium carbonate. It achieves therapeutic effects by employing GOD to disrupt glycolysis and increase oxidative stress, while calcium carbonate promotes Ca2+-mediated mitochondrial dysfunction. This dual approach effectively reduces ATP production and lowers heat resistance in tumor cells, thereby improving the effectiveness of PTT against tumors [144]. Notably, an abraxane-like nanoplatform named LCIR effectively depletes ATP by inhibiting mitochondrial complexes and hexokinase II, enhancing NIR-triggered photodynamic and photothermal treatments. This approach significantly reduces tumor size with minimal systemic toxicity, indicating its potential to overcome resistance in conventional cancer therapies [145]. Besides, a novel organic nanocarrier DA-P-SS-T/PTX, modified with acid-cleavable dimethylmaleic anhydride and conjugated with mitochondria-targeting TPP, demonstrates enhanced cellular uptake and specific targeting to mitochondria in tumor environments. It facilitates prolonged blood circulation, effectively targets the mitochondrial outer membrane in tumor cells, leading to decreased membrane potential and ATP levels, thereby inhibiting P-glycoprotein and curtailing both cancer drug resistance and metastasis [193, 194].
Conclusions
In summary, mitochondria are central to cellular energy processes, and their dysfunction is closely associated with cancer development and treatment outcomes. The focus on using multifunctional nanosystems to target mitochondrial metabolism in tumor cells represents a promising approach in cancer therapy. This innovative direction exploits the specific metabolic vulnerabilities of cancer cells, potentially leading to more effective and targeted treatments. Advances in nanomedicine targeting mitochondrial pathways may revolutionize cancer therapy and have implications for managing other diseases characterized by mitochondrial dysregulation.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- OMM:
-
Outer mitochondrial membrane
- IMS:
-
Intermembrane space
- IMM:
-
Inner mitochondrial membrane
- MM:
-
Mitochondrial matrix
- Acetyl-CoA:
-
Acetyl coenzyme A
- TCA:
-
Tricarboxylic acid
- OXPHOS:
-
Oxidative phosphorylation
- FAO:
-
Fatty acid oxidation
- Gln:
-
Glutamine
- ROS:
-
Reactive oxygen species
- MtDNA:
-
Mitochondrial DNA
- ETC:
-
Electron transport chain
- CPT:
-
Carnitine palmitoyltransferase
- Glu:
-
Glutamate
- GSH:
-
Glutathione
- GLS:
-
Glutaminase
- GDH:
-
Glutamate dehydrogenase
- RNS:
-
Reactive nitrogen species
- PTT:
-
Photothermal therapy
- PDT:
-
Photodynamic therapy
References
Antonicka H, Lin ZY, Janer A, Aaltonen MJ, Weraarpachai W, Gingras AC, et al. A high-density human mitochondrial proximity Interaction Network. Cell Metab. 2020;32(3):479–97. e9.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–59.
Bozi LHM, Campos JC, Zambelli VO, Ferreira ND, Ferreira JCB. Mitochondrially-targeted treatment strategies. Mol Aspects Med. 2020;71:100836.
Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283(5407):1482–8.
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18(4):243–58.
Zhang X, **e F, Ma S, Ma C, Jiang X, Yi Y, et al. Mitochondria: one of the vital hubs for molecular hydrogen’s biological functions. Front Cell Dev Biol. 2023;11:1283820.
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31.
** P, Jiang J, Zhou L, Huang Z, Nice EC, Huang C, et al. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J Hematol Oncol. 2022;15(1):97.
Scagliola A, Mainini F, Cardaci S. The Tricarboxylic Acid cycle at the crossroad between Cancer and Immunity. Antioxid Redox Signal. 2020;32(12):834–52.
Murphy MP, O’Neill LAJ. Krebs cycle reimagined: the emerging roles of Succinate and Itaconate as Signal transducers. Cell. 2018;174(4):780–4.
Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102.
Pokharel MD, Marciano DP, Fu P, Franco MC, Unwalla H, Tieu K, et al. Metabolic reprogramming, oxidative stress, and pulmonary hypertension. Redox Biol. 2023;64:102797.
Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O’Neill LA, et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. 2019;1:16–33.
Murphy MP, Chouchani ET. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat Chem Biol. 2022;18(5):461–9.
Sazanov LA. A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol. 2015;16(6):375–88.
Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol. 2022;23(2):141–61.
Neupane P, Bhuju S, Thapa N, Bhattarai HK. ATP synthase: structure, function and inhibition. Biomol Concepts. 2019;10(1):1–10.
Gupta N, Verma K, Nalla S, Kulshreshtha A, Lall R, Prasad S. Free radicals as a double-edged Sword: the Cancer Preventive and therapeutic roles of Curcumin. Molecules. 2020;25(22).
Moldogazieva NT, Lutsenko SV, Terentiev AA. Reactive oxygen and Nitrogen species-Induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78(21):6040–7.
Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289.
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS et al. Oxidative stress in Human Pathology and Aging: Molecular mechanisms and perspectives. Cells. 2022;11(3).
Weindel CG, Martinez EL, Zhao X, Mabry CJ, Bell SL, Vail KJ et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell. 2022;185(17):3214-31 e23.
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021;41:101947.
Yang ZJ, Zhao CL, Liang WQ, Chen ZR, Du ZD, Gong SS. ROS-induced oxidative stress and mitochondrial dysfunction: a possible mechanism responsible for noise-induced ribbon synaptic damage. Am J Transl Res. 2024;16(1):272–84.
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–9.
Houten SM, Violante S, Ventura FV, Wanders RJ. The Biochemistry and Physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu Rev Physiol. 2016;78:23–44.
Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 2014;34:1–30.
Kim JA. Peroxisome metabolism in Cancer. Cells. 2020;9(7).
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(2):113.
Panieri E, Telkoparan-Akillilar P, Suzen S, Saso L. The NRF2/KEAP1 Axis in the regulation of Tumor Metabolism: mechanisms and therapeutic perspectives. Biomolecules. 2020;10(5).
Garcia-Bermudez J, Williams RT, Guarecuco R, Birsoy K. Targeting extracellular nutrient dependencies of cancer cells. Mol Metab. 2020;33:67–82.
Mates JM, Campos-Sandoval JA, Santos-Jimenez JL, Marquez J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019;467:29–39.
Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int J Mol Med. 2019;44(1):3–15.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–50.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83.
Lennicke C, Rahn J, Lichtenfels R, Wessjohann LA, Seliger B. Hydrogen peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun Signal. 2015;13:39.
Raimondi V, Ciccarese F, Ciminale V. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br J Cancer. 2020;122(2):168–81.
Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–9.
Parekh A, Das S, Parida S, Das CK, Dutta D, Mallick SK, et al. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene. 2018;37(33):4546–61.
Tyagi A, Haq S, Ramakrishna S. Redox regulation of DUBs and its therapeutic implications in cancer. Redox Biol. 2021;48:102194.
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev. 2016;2016:4350965.
Sainero-Alcolado L, Liano-Pons J, Ruiz-Perez MV, Arsenian-Henriksson M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022;29(7):1304–17.
Pavlova NN, Thompson CB. The emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23(1):27–47.
Kodama M, Oshikawa K, Shimizu H, Yoshioka S, Takahashi M, Izumi Y, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat Commun. 2020;11(1):1320.
Kawamura T, Takehora Y, Hori N, Takakura Y, Yamaguchi N, Takano H, et al. VGLL3 increases the dependency of cancer cells on de novo nucleotide synthesis through GART expression. J Cell Biochem. 2022;123(6):1064–76.
Bian X, Liu R, Meng Y, **ng D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218(1).
Padanad MS, Konstantinidou G, Venkateswaran N, Melegari M, Rindhe S, Mitsche M, et al. Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep. 2016;16(6):1614–28.
Wright HJ, Hou J, Xu B, Cortez M, Potma EO, Tromberg BJ, et al. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci U S A. 2017;114(32):E6556–65.
Bi J, Mischel PS. Acyl-CoA-Binding protein fuels gliomagenesis. Cell Metab. 2019;30(2):229–30.
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, et al. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett. 2018;435:92–100.
Shi J, Fu H, Jia Z, He K, Fu L, Wang W. High expression of CPT1A predicts adverse outcomes: a potential therapeutic target for Acute myeloid leukemia. EBioMedicine. 2016;14:55–64.
Liu PP, Liu J, Jiang WQ, Carew JS, Ogasawara MA, Pelicano H, et al. Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline. Oncogene. 2016;35(43):5663–73.
Tang M, Dong X, **ao L, Tan Z, Luo X, Yang L, et al. CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma. Cell Death Dis. 2022;13(4):331.
Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22(4):427–32.
Quan J, Li N, Tan Y, Liu H, Liao W, Cao Y, et al. PGC1alpha-mediated fatty acid oxidation promotes TGFbeta1-induced epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma. Life Sci. 2022;300:120558.
Yan X, Zhang G, Bie F, Lv Y, Ma Y, Ma M, et al. Eugenol inhibits oxidative phosphorylation and fatty acid oxidation via downregulation of c-Myc/PGC-1beta/ERRalpha signaling pathway in MCF10A-ras cells. Sci Rep. 2017;7(1):12920.
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-Regulated fatty acid beta-oxidation is critical for breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27(1):136–50. e5.
Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5(1):108.
Yang M, Liu K, Chen P, Zhu H, Wang J, Huang J. Bromodomain-containing protein 4 (BRD4) as an epigenetic regulator of fatty acid metabolism genes and ferroptosis. Cell Death Dis. 2022;13(10):912.
Yuan X, Kang Y, Dong J, Li R, Ye J, Fan Y, et al. Self-triggered thermoelectric nanoheterojunction for cancer catalytic and immunotherapy. Nat Commun. 2023;14(1):5140.
Kang Y, Xu L, Dong J, Yuan X, Ye J, Fan Y, et al. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat Commun. 2024;15(1):1042.
Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–72.
Barry NP, Sadler PJ. Challenges for metals in medicine: how nanotechnology may help to shape the future. ACS Nano. 2013;7(7):5654–9.
Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–108.
Bockamp E, Rosigkeit S, Siegl D, Schuppan D. Nano-enhanced Cancer Immunotherapy: Immunology Encounters Nanotechnology. Cells. 2020;9(9).
Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79.
Samaridou E, Heyes J, Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev. 2020;154–155:37–63.
Moradi Kashkooli F, Soltani M, Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J Control Release. 2020;327:316–49.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Chen L, Mao Z, Wang Y, Kang Y, Wang Y, Mei L, et al. Edge modification facilitated heterogenization and exfoliation of two-dimensional nanomaterials for cancer catalytic therapy. Sci Adv. 2022;8(39):eabo7372.
Huang L, Zhao S, Fang F, Xu T, Lan M, Zhang J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials. 2021;268:120557.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–17.
Hou YC, Zhang C, Zhang ZJ, **a L, Rao KQ, Gu LH, et al. Aggregation-Induced Emission (AIE) and magnetic resonance imaging characteristics for targeted and image-guided siRNA therapy of Hepatocellular Carcinoma. Adv Healthc Mater. 2022;11(17):e2200579.
Jain P, Kathuria H, Momin M. Clinical therapies and nano drug delivery systems for urinary bladder cancer. Pharmacol Ther. 2021;226:107871.
Xu M, Li S. Nano-drug delivery system targeting tumor microenvironment: a prospective strategy for melanoma treatment. Cancer Lett. 2023;574:216397.
Xu C, Nam J, Hong H, Xu Y, Moon JJ. Positron Emission Tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for Personalized Cancer Immunotherapy. ACS Nano. 2019;13(10):12148–61.
Zhang Q, Kuang G, Wang H, Zhao Y, Wei J, Shang L. Multi-bioinspired MOF Delivery systems from Microfluidics for Tumor Multimodal Therapy. Adv Sci (Weinh). 2023;10(33):e2303818.
Estape Senti M, Garcia Del Valle L, Schiffelers RM. mRNA delivery systems for cancer immunotherapy: lipid nanoparticles and beyond. Adv Drug Deliv Rev. 2024;206:115190.
Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119(8).
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15.
AlQahtani SA, Harisa GI, Alomrani AH, Alanazi FK, Badran MM. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf B Biointerfaces. 2021;197:111380.
Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11(13):6370–92.
Xu S, Cui K, Long K, Li J, Fan N, Lam WC, et al. Red Light-triggered anti-angiogenic and photodynamic combination therapy of age-related Macular Degeneration. Adv Sci (Weinh). 2023;10(31):e2301985.
Alamzadeh Z, Beik J, Pirhajati Mahabadi V, Abbasian Ardekani A, Ghader A, Kamrava SK, et al. Ultrastructural and optical characteristics of cancer cells treated by a nanotechnology based chemo-photothermal therapy method. J Photochem Photobiol B. 2019;192:19–25.
Li J, Wang Q, **a G, Adilijiang N, Li Y, Hou Z et al. Recent advances in targeted drug delivery strategy for enhancing Oncotherapy. Pharmaceutics. 2023;15(9).
Vilimas T, Wang AQ, Patnaik S, Hughes EA, Singleton MD, Knotts Z, et al. Pharmacokinetic evaluation of the PNC disassembler metarrestin in wild-type and Pdx1-Cre;LSL-Kras(G12D/+);Tp53(R172H/+) (KPC) mice, a genetically engineered model of pancreatic cancer. Cancer Chemother Pharmacol. 2018;82(6):1067–80.
Liu M, Peng Y, Nie Y, Liu P, Hu S, Ding J, et al. Co-delivery of doxorubicin and DNAzyme using ZnO@polydopamine core-shell nanocomposites for chemo/gene/photothermal therapy. Acta Biomater. 2020;110:242–53.
Di Nottia M, Verrigni D, Torraco A, Rizza T, Bertini E, Carrozzo R. Mitochondrial dynamics: Molecular mechanisms, related primary mitochondrial disorders and therapeutic approaches. Genes (Basel). 2021;12(2).
Li C, Zhang W, Liu S, Hu X, **e Z. Mitochondria-Targeting Organic nanoparticles for enhanced Photodynamic/Photothermal therapy. ACS Appl Mater Interfaces. 2020;12(27):30077–84.
Tan Y, Zhu Y, Zhao Y, Wen L, Meng T, Liu X, et al. Mitochondrial alkaline pH-responsive drug release mediated by Celastrol loaded glycolipid-like micelles for cancer therapy. Biomaterials. 2018;154:169–81.
Liu Y, Zhou Z, Hou J, **ong W, Kim H, Chen J, et al. Tumor Selective metabolic reprogramming as a prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv Mater. 2022;34(41):e2206121.
Zhang L, Wang D, Yang K, Sheng D, Tan B, Wang Z, et al. Mitochondria-targeted Artificial Nano-RBCs for amplified synergistic Cancer phototherapy by a single NIR Irradiation. Adv Sci (Weinh). 2018;5(8):1800049.
Jiang L, Li L, He X, Yi Q, He B, Cao J, et al. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials. 2015;52:126–39.
Zhu XJ, Li RF, Xu L, Yin H, Chen L, Yuan Y, et al. A novel self-assembled Mitochondria-Targeting protein nanoparticle acting as Theranostic platform for Cancer. Small. 2019;15(2):e1803428.
Kim HR, Cho HB, Lee S, Park JI, Kim HJ, Park KH. Fusogenic liposomes encapsulating mitochondria as a promising delivery system for osteoarthritis therapy. Biomaterials. 2023;302:122350.
Cao S, **a Y, Shao J, Guo B, Dong Y, Pijpers IAB, et al. Biodegradable polymersomes with structure inherent fluorescence and targeting capacity for enhanced photo-dynamic therapy. Angew Chem Int Ed Engl. 2021;60(32):17629–37.
Bao W, Liu M, Meng J, Liu S, Wang S, Jia R, et al. MOFs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload. Nat Commun. 2021;12(1):6399.
Yoong SL, Wong BS, Zhou QL, Chin CF, Li J, Venkatesan T, et al. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials. 2014;35(2):748–59.
Yang X, Wang D, Zhu J, Xue L, Ou C, Wang W, et al. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem Sci. 2019;10(13):3779–85.
Lin LS, Wang JF, Song J, Liu Y, Zhu G, Dai Y, et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics. 2019;9(24):7200–9.
Hsieh CH, Hsieh HC, Shih FS, Wang PW, Yang LX, Shieh DB, et al. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11(14):7072–91.
Sun Z, Chen W, Liu J, Yu B, Jiang C, Lu L. Mitochondria-Targeting enhanced phototherapy by intrinsic characteristics Engineered one-for-all nanoparticles. ACS Appl Mater Interfaces. 2021;13(30):35568–78.
Sun L, Liu Y, Yang N, Ye X, Liu Z, Wu J, et al. Gold nanoparticles inhibit tumor growth via targeting the Warburg effect in a c-Myc-dependent way. Acta Biomater. 2023;158:583–98.
Hernandes EP, Lazarin-Bidoia D, Bini RD, Nakamura CV, Cotica LF, de Oliveira Silva Lautenschlager S. Doxorubicin-loaded Iron oxide nanoparticles induce oxidative stress and cell cycle arrest in breast Cancer cells. Antioxid (Basel). 2023;12(2).
Jahanbani J, Ghotbi M, Shahsavari F, Seydi E, Rahimi S, Pourahmad J. Selective anticancer activity of superparamagnetic iron oxide nanoparticles (SPIONs) against oral tongue cancer using in vitro methods: the key role of oxidative stress on cancerous mitochondria. J Biochem Mol Toxicol. 2020;34(10):e22557.
Jiang F, Lee C, Zhang W, Jiang W, Cao Z, Chong HB, et al. Radiodynamic therapy with CsI(na)@MgO nanoparticles and 5-aminolevulinic acid. J Nanobiotechnol. 2022;20(1):330.
Li Z, Guo D, Yin X, Ding S, Shen M, Zhang R, et al. Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/Caspase-9/Caspase-3 signaling pathway in vitro. Biomed Pharmacother. 2020;122:109712.
Singh N, Kim J, Kim J, Lee K, Zunbul Z, Lee I, et al. Covalent organic framework nanomedicines: Biocompatibility for advanced nanocarriers and cancer theranostics applications. Bioact Mater. 2023;21:358–80.
Zhang Y, Jia Q, Nan F, Wang J, Liang K, Li J, et al. Carbon dots nanophotosensitizers with tunable reactive oxygen species generation for mitochondrion-targeted type I/II photodynamic therapy. Biomaterials. 2023;293:121953.
Zuo L, Nie W, Yu S, Zhuang WR, Liang C, Li S, et al. Biomimetic nanovesicle with Mitochondria-Synthesized Sonosensitizer and Mitophagy Inhibition for Cancer Sono-Immunotherapy. Nano Lett. 2023;23(7):3005–13.
Dai W, Deng Y, Chen X, Huang Y, Hu H, ** Q, et al. A mitochondria-targeted supramolecular nanoplatform for peroxynitrite-potentiated oxidative therapy of orthotopic hepatoma. Biomaterials. 2022;290:121854.
Zha S, Liu H, Li H, Li H, Wong KL, All AH. Functionalized nanomaterials capable of crossing the blood-brain barrier. ACS Nano. 2024;18(3):1820–45.
**ong H, Ye J, Wang M, Wang Y, Liu X, Jiang H, et al. In-situ bio-assembled specific au NCs-Aptamer-pyro conjugates nanoprobe for tumor imaging and mitochondria-targeted photodynamic therapy. Biosens Bioelectron. 2022;218:114763.
Zhang J, Yin X, Li C, Yin X, Xue Q, Ding L, et al. A multifunctional Photoacoustic/Fluorescence Dual-Mode-Imaging Gold-based Theranostic Nanoformulation without External Laser limitations. Adv Mater. 2022;34(19):e2110690.
Gong N, Ma X, Ye X, Zhou Q, Chen X, Tan X, et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat Nanotechnol. 2019;14(4):379–87.
Liu B, Bian Y, Liang S, Yuan M, Dong S, He F, et al. One-step integration of Tumor Microenvironment-Responsive Calcium and Copper Peroxides Nanocomposite for enhanced Chemodynamic/Ion-Interference therapy. ACS Nano. 2022;16(1):617–30.
Xu S, Zhang P, Heing-Becker I, Zhang J, Tang P, Bej R, et al. Dual tumor- and subcellular-targeted photodynamic therapy using glucose-functionalized MoS(2) nanoflakes for multidrug-resistant tumor ablation. Biomaterials. 2022;290:121844.
Qi J, **ong Y, Cheng K, Huang Q, Cao J, He F, et al. Heterobifunctional PEG-grafted black phosphorus quantum dots: three-in-one nano-platforms for mitochondria-targeted photothermal cancer therapy. Asian J Pharm Sci. 2021;16(2):222–35.
Zhou Z, Liu Y, Song W, Jiang X, Deng Z, **ong W, et al. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J Control Release. 2022;352:793–812.
Mittal L, Camarillo IG, Varadarajan GS, Srinivasan H, Aryal UK, Sundararajan R. High-throughput, label-free quantitative proteomic studies of the Anticancer effects of Electrical pulses with Turmeric Silver nanoparticles: an in vitro Model Study. Sci Rep. 2020;10(1):7258.
Zhou Z, Vazquez-Gonzalez M, Willner I. Stimuli-responsive metal-organic framework nanoparticles for controlled drug delivery and medical applications. Chem Soc Rev. 2021;50(7):4541–63.
Lv C, Kang W, Liu S, Yang P, Nishina Y, Ge S, et al. Growth of ZIF-8 nanoparticles in situ on Graphene Oxide nanosheets: a multifunctional nanoplatform for combined Ion-Interference and Photothermal Therapy. ACS Nano. 2022;16(7):11428–43.
Yang F, Yu W, Yu Q, Liu X, Liu C, Lu C, et al. Mitochondria-targeted nanosystem with reactive oxygen species-controlled release of CO to enhance photodynamic therapy of PCN-224 by Sensitizing Ferroptosis. Small. 2023;19(16):e2206124.
Wu J, Ning P, Gao R, Feng Q, Shen Y, Zhang Y, et al. Programmable ROS-Mediated Cancer Therapy via Magneto-inductions. Adv Sci (Weinh). 2020;7(12):1902933.
Liu T, **ong CF, Zhang LJ, Jiao GH, Shi H, Feng J, et al. Boosting Doxorubicin-Induced Mitochondria apoptosis for the monodrug-mediated combination of Chemotherapy and Chemodynamic Therapy. Adv Healthc Mater. 2023;12(3):e2202045.
Cheng Y, Ji Y, Tong J. Triple stimuli-responsive supramolecular nanoassembly with mitochondrial targetability for chemophotothermal therapy. J Control Release. 2020;327:35–49.
Wu S, Xu L, He C, Wang P, Qin J, Guo F, et al. Lactate Efflux inhibition by Syrosingopine/LOD co-loaded Nanozyme for Synergetic Self-Replenishing Catalytic Cancer Therapy and Immune Microenvironment Remodeling. Adv Sci (Weinh). 2023;10(26):e2300686.
Hu H, Deng X, Song Q, Yang W, Zhang Y, Liu W, et al. Mitochondria-targeted accumulation of oxygen-irrelevant free radicals for enhanced synergistic low-temperature photothermal and thermodynamic therapy. J Nanobiotechnol. 2021;19(1):390.
Truong Hoang Q, Huynh KA, Nguyen Cao TG, Kang JH, Dang XN, Ravichandran V, et al. Piezocatalytic 2D WS(2) nanosheets for Ultrasound-Triggered and Mitochondria-targeted Piezodynamic Cancer Therapy Synergized with Energy metabolism-targeted chemotherapy. Adv Mater. 2023;35(18):e2300437.
Zhou Z, Zheng C, Liu Y, Luo W, Deng H, Shen J. Chitosan biguanide induced mitochondrial inhibition to amplify the efficacy of oxygen-sensitive tumor therapies. Carbohydr Polym. 2022;295:119878.
Yang Z, Wang J, Liu S, Li X, Miao L, Yang B, et al. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway. Biomaterials. 2020;229:119580.
Zhang J, Yan L, Wei P, Zhou R, Hua C, **ao M, et al. PEG-GO@XN nanocomposite suppresses breast cancer metastasis via inhibition of mitochondrial oxidative phosphorylation and blockade of epithelial-to-mesenchymal transition. Eur J Pharmacol. 2021;895:173866.
Guo W, Chen Z, Feng X, Shen G, Huang H, Liang Y, et al. Graphene oxide (GO)-based nanosheets with combined chemo/photothermal/photodynamic therapy to overcome gastric cancer (GC) paclitaxel resistance by reducing mitochondria-derived adenosine-triphosphate (ATP). J Nanobiotechnol. 2021;19(1):146.
Hu XK, Rao SS, Tan YJ, Yin H, Luo MJ, Wang ZX, et al. Fructose-coated Angstrom silver inhibits osteosarcoma growth and metastasis via promoting ROS-dependent apoptosis through the alteration of glucose metabolism by inhibiting PDK. Theranostics. 2020;10(17):7710–29.
Bhattacharya SR, Bhattacharya K, Xavier VJ, Ziarati A, Picard D, Burgi T. The Atomically Precise Gold/Captopril nanocluster au(25)(Capt)(18) gains anticancer activity by inhibiting mitochondrial oxidative phosphorylation. ACS Appl Mater Interfaces. 2022;14(26):29521–36.
Zhou W, Chen S, Ouyang Y, Huang B, Zhang H, Zhang W, et al. A supramolecular nanoplatform for imaging-guided phototherapies via hypoxia tumour microenvironment remodeling. Chem Sci. 2023;14(41):11481–9.
Lu L, Liu G, Lin C, Li K, He T, Zhang J, et al. Mitochondrial metabolism targeted nanoplatform for efficient triple-negative breast Cancer Combination Therapy. Adv Healthc Mater. 2021;10(20):e2100978.
Zou Y, Sun Y, Wang Y, Zhang D, Yang H, Wang X, et al. Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment. Nat Commun. 2023;14(1):4557.
Zhang K, Zhu J, Wang R, Zhu W, Zhang Z, Gong L, et al. Mitochondria-anchoring self-assembled nanoparticles for multi-path energy depletion: a nano bomb in chemo-co-starvation therapy. Int J Pharm. 2023;642:123180.
Yu X, Lyu M, Ou X, Liu W, Yang X, Ma X, et al. AIEgens/Mitochondria Nanohybrids as Bioactive Microwave Sensitizers for Non-thermal Microwave Cancer Therapy. Adv Healthc Mater. 2023;12(12):e2202907.
Liu J, Liu X, Wu M, Qi G, Liu B. Engineering Living Mitochondria with AIE Photosensitizer for Synergistic Cancer cell ablation. Nano Lett. 2020;20(10):7438–45.
Zeng WN, Yu QP, Wang D, Liu JL, Yang QJ, Zhou ZK, et al. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. J Nanobiotechnol. 2021;19(1):79.
Zhang H, Liu R, Wan P, You X, Li S, Liu Z, et al. Targeting tumor energy metabolism via simultaneous inhibition of mitochondrial respiration and glycolysis using biodegradable hydroxyapatite nanorods. Colloids Surf B Biointerfaces. 2023;226:113330.
Ding XL, Liu MD, Cheng Q, Guo WH, Niu MT, Huang QX, et al. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy. Biomaterials. 2022;281:121369.
Le XT, Lee J, Nguyen NT, Lee WT, Lee ES, Oh KT, et al. Combined phototherapy with metabolic reprogramming-targeted albumin nanoparticles for treating breast cancer. Biomater Sci. 2022;10(24):7117–32.
Wang H, Shi W, Zeng D, Huang Q, **e J, Wen H, et al. pH-activated, mitochondria-targeted, and redox-responsive delivery of paclitaxel nanomicelles to overcome drug resistance and suppress metastasis in lung cancer. J Nanobiotechnol. 2021;19(1):152.
Li F, Liu Y, Dong Y, Chu Y, Song N, Yang D. Dynamic assembly of DNA nanostructures in living cells for mitochondrial interference. J Am Chem Soc. 2022;144(10):4667–77.
Dahabiyeh LA, Mahmoud NN, Al-Natour MA, Safo L, Kim DH, Khalil EA et al. Phospholipid-gold nanorods induce Energy Crisis in MCF-7 cells: cytotoxicity evaluation using LC-MS-Based Metabolomics Approach. Biomolecules. 2021;11(3).
Wang YY, Zhang XY, Li SL, Jiang FL, Jiang P, Liu Y. AuPt-Loaded Cu-Doped polydopamine nanocomposites with multienzyme-mimic activities for dual-modal imaging-guided and cuproptosis-enhanced Photothermal/Nanocatalytic therapy. Anal Chem. 2023;95(37):14025–35.
Jiang Y, Tan Y, **ao K, Li X, Shao K, Song J, et al. pH-Regulating nanoplatform for the Double Channel Chase of Tumor Cells by the Synergistic Cascade between Chlorine Treatment and Methionine-Depletion Starvation Therapy. ACS Appl Mater Interfaces. 2021;13(46):54690–705.
She W, Li H, Wang Z, Liu T, Zhao D, Guo Z, et al. Site-specific controlled-release nanoparticles for immune reprogramming via dual metabolic inhibition against triple-negative breast cancer. J Control Release. 2024;366:204–20.
Nguyen Cao TG, Truong Hoang Q, Kang JH, Kang SJ, Ravichandran V, Rhee WJ, et al. Bioreducible exosomes encapsulating glycolysis inhibitors potentiate mitochondria-targeted sonodynamic cancer therapy via cancer-targeted drug release and cellular energy depletion. Biomaterials. 2023;301:122242.
Yang R, Fang XL, Zhen Q, Chen QY, Feng C. Mitochondrial targeting nano-curcumin for attenuation on PKM2 and FASN. Colloids Surf B Biointerfaces. 2019;182:110405.
Wang J, Zhang L, **n H, Guo Y, Zhu B, Su L, et al. Mitochondria-targeting folic acid-modified nanoplatform based on mesoporous carbon and a bioactive peptide for improved colorectal cancer treatment. Acta Biomater. 2022;152:453–72.
Liu X, Li Y, Wang K, Chen Y, Shi M, Zhang X, et al. GSH-Responsive Nanoprodrug to inhibit glycolysis and alleviate immunosuppression for Cancer Therapy. Nano Lett. 2021;21(18):7862–9.
Yang T, Zhang X, Yang X, Li Y, **ang J, **ang C, et al. A mitochondria-targeting self-assembled carrier-free lonidamine nanodrug for redox-activated drug release to enhance cancer chemotherapy. J Mater Chem B. 2023;11(17):3951–7.
Machuca A, Garcia-Calvo E, Anunciacao DS, Luque-Garcia JL. Integration of Transcriptomics and Metabolomics to reveal the Molecular mechanisms Underlying Rhodium nanoparticles-based photodynamic Cancer therapy. Pharmaceutics. 2021;13(10).
Zhang Y, Ren Y, Xu H, Li L, Qian F, Wang L, et al. Cascade-Responsive 2-DG Nanocapsules encapsulate aV-siCPT1C conjugates to inhibit Glioblastoma through multiple inhibition of Energy Metabolism. ACS Appl Mater Interfaces. 2023;15(8):10356–70.
Gao Y, Song Z, Jia L, Tang Y, Wang C, Zhao X, et al. Self-amplified ROS production from fatty acid oxidation enhanced tumor immunotherapy by atorvastatin/PD-L1 siRNA lipopeptide nanoplexes. Biomaterials. 2022;291:121902.
Wang H, Lin M, Chen G, **ao Z, Shuai X. Nanodrug regulates ROS homeostasis via enhancing fatty acid oxidation and inhibiting autophagy to overcome tumor drug resistance. Biomater Sci. 2023;11(21):7179–87.
Luo L, Li X, Zhang J, Zhu C, Jiang M, Luo Z, et al. Enhanced immune memory through a constant photothermal-metabolism regulation for cancer prevention and treatment. Biomaterials. 2021;270:120678.
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–33.
Mullen AR, Wheaton WW, ** ES, Chen PH, Sullivan LB, Cheng T, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481(7381):385–8.
Tang E, Liu S, Zhang Z, Zhang R, Huang D, Gao T, et al. Therapeutic potential of glutamine pathway in Lung Cancer. Front Oncol. 2021;11:835141.
Ren J, Zhou J, Liu H, Jiao X, Cao Y, Xu Z, et al. Ultrasound (US)-activated redox dyshomeostasis therapy reinforced by immunogenic cell death (ICD) through a mitochondrial targeting liposomal nanosystem. Theranostics. 2021;11(19):9470–91.
Wang Q, Li S, Xu C, Hua A, Wang C, **ong Y, et al. A novel lonidamine derivative targeting mitochondria to eliminate cancer stem cells by blocking glutamine metabolism. Pharmacol Res. 2023;190:106740.
Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102(2):893–992.
Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19(11):713–30.
Zheng P, Ding B, Shi R, Jiang Z, Xu W, Li G, et al. A multichannel ca(2+) Nanomodulator for Multilevel mitochondrial Destruction-mediated Cancer Therapy. Adv Mater. 2021;33(15):e2007426.
Shao F, Han J, Tian Z, Wang Z, Liu S, Wu Y. Synergistic ROS generation and directional overloading of endogenous calcium induce mitochondrial dysfunction in living cells. Biomaterials. 2023;301:122284.
Wang X, Li Y, Deng X, Jia F, Cui X, Lu J, et al. Colloidally stabilized DSPE-PEG-Glucose/Calcium phosphate hybrid nanocomposites for enhanced photodynamic Cancer Therapy via complementary mitochondrial ca(2+) overload and autophagy inhibition. ACS Appl Mater Interfaces. 2021;13(33):39112–25.
Ahmad A, Rashid S, Chaudhary AA, Alawam AS, Alghonaim MI, Raza SS, et al. Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin Cancer Biol. 2023;89:38–60.
Kang Y, Mao Z, Wang Y, Pan C, Ou M, Zhang H, et al. Design of a two-dimensional interplanar heterojunction for catalytic cancer therapy. Nat Commun. 2022;13(1):2425.
Gomes A, Sengupta J, Datta P, Ghosh S, Gomes A. Physiological interactions of nanoparticles in Energy Metabolism, Immune function and their Biosafety: a review. J Nanosci Nanotechnol. 2016;16(1):92–116.
Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Gonzalez-Valdivieso J, Girotti A, Schneider J, Arias FJ. Advanced nanomedicine and cancer: challenges and opportunities in clinical translation. Int J Pharm. 2021;599:120438.
** X, Yang H, Mao Z, Wang B. Cathepsin B-responsive multifunctional peptide conjugated gold nanorods for mitochondrial targeting and precise photothermal cancer therapy. J Colloid Interface Sci. 2021;601:714–26.
Yang GG, Pan ZY, Zhang DY, Cao Q, Ji LN, Mao ZW. Precisely assembled nanoparticles against Cisplatin Resistance via Cancer-Specific Targeting of Mitochondria and Imaging-guided chemo-photothermal therapy. ACS Appl Mater Interfaces. 2020;12(39):43444–55.
**ao Y, Zhang T, Ma X, Yang QC, Yang LL, Yang SC, et al. Microenvironment-responsive Prodrug-Induced pyroptosis boosts Cancer Immunotherapy. Adv Sci (Weinh). 2021;8(24):e2101840.
Shen R, Jiang Q, Li P, Wang D, Yu C, Meng T, et al. Targeted plus controlled - composite nano delivery system opens the tumor vascular and microenvironment normalization window for anti-tumor therapy. Int J Pharm. 2023;647:123512.
Kang X, Bu F, Feng W, Liu F, Yang X, Li H, et al. Dual-Cascade Responsive nanoparticles enhance pancreatic Cancer Therapy by eliminating tumor-resident intracellular Bacteria. Adv Mater. 2022;34(49):e2206765.
Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci U S A. 2016;113(36):E5328–36.
Schlaepfer IR, Rider L, Rodrigues LU, Gijon MA, Pac CT, Romero L, et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13(10):2361–71.
Missiroli S, Perrone M, Genovese I, Pinton P, Giorgi C. Cancer metabolism and mitochondria: finding novel mechanisms to fight tumours. EBioMedicine. 2020;59:102943.
Delaunay S, Pascual G, Feng B, Klann K, Behm M, Hotz-Wagenblatt A, et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature. 2022;607(7919):593–603.
Liu Y, Chen C, Wang X, Sun Y, Zhang J, Chen J, et al. An epigenetic role of Mitochondria in Cancer. Cells. 2022;11:16.
Izzo V, Bravo-San Pedro JM, Sica V, Kroemer G, Galluzzi L. Mitochondrial permeability transition: New findings and persisting uncertainties. Trends Cell Biol. 2016;26(9):655–67.
Bonora M, Wieckowsk MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34(12):1608.
Szabo L, Cummins N, Paganetti P, Odermatt A, Papassotiropoulos A, Karch C, et al. ER-mitochondria contacts and cholesterol metabolism are disrupted by disease-associated tau protein. EMBO Rep. 2023;24(8):e57499.
Fransen M, Lismont C, Walton P. The peroxisome-mitochondria connection: how and why? Int J Mol Sci. 2017;18(6).
de Montes P. Mitochondria-plasma membrane interactions and communication. J Biol Chem. 2021;297(4):101164.
Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of Nanoparticulate nanomedicines: pathways for Translational Development and Commercialization. Front Pharmacol. 2018;9:790.
Wen R, Banik B, Pathak RK, Kumar A, Kolishetti N, Dhar S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv Drug Deliv Rev. 2016;99(Pt A):52–69.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (81901006, 82372905), Young Top-notch Talent of Pearl River Talent Plan (0920220228), Guangdong Provincial Science and Technology Project Foundation (2022A0505050038), and Science Research Cultivation Program of Stomatological Hospital, Southern Medical University (PY2020002, PY2022019).
Author information
Authors and Affiliations
Contributions
LC, and XZ conceived of the presented idea. LC, PL, XZ, YL, YFL and JZ wrote the manuscript. PL and YL created the graphs. All authors contributed to and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, P., Lu, Y., Zheng, J. et al. Strategic disruption of cancer’s powerhouse: precise nanomedicine targeting of mitochondrial metabolism. J Nanobiotechnol 22, 318 (2024). https://doi.org/10.1186/s12951-024-02585-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02585-3