Abstract
Background
Myocardial infarction (MI), a representative form of ischemic heart disease, remains a huge burden worldwide. This study aimed to explore whether extracellular vesicles (EVs) secreted from hyaluronic acid (HA)-primed induced mesenchymal stem cells (HA-iMSC-EVs) could enhance the cardiac repair after MI.
Results
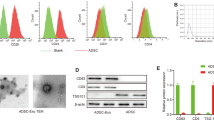
HA-iMSC-EVs showed typical characteristics for EVs such as morphology, size, and marker proteins expression. Compared with iMSC-EVs, HA-iMSC-EVs showed enhanced tube formation and survival against oxidative stress in endothelial cells, while reduced reactive oxygen species (ROS) generation in cardiomyocytes. In THP-1 macrophages, both types of EVs markedly reduced the expression of pro-inflammatory signaling players, whereas HA-iMSC-EVs were more potent in augmenting anti-inflammatory markers. A significant decrease of inflammasome proteins was observed in HA-iMSC-EV-treated THP-1. Further, phospho-SMAD2 as well as fibrosis markers in TGF-β1-stimulated cardiomyocytes were reduced in HA-iMSC-EVs treatment. Proteomic data showed that HA-iMSC-EVs were enriched with multiple pathways including immunity, extracellular matrix organization, angiogenesis, and cell cycle. The localization of HA-iMSC-EVs in myocardium was confirmed after delivery by either intravenous or intramyocardial route, with the latter increased intensity. Echocardiography revealed that intramyocardial HA-iMSC-EVs injections improved cardiac function and reduced adverse cardiac remodeling and necrotic size in MI heart. Histologically, MI hearts receiving HA-iMSC-EVs had increased capillary density and viable myocardium, while showed reduced fibrosis.
Conclusions
Our results suggest that HA-iMSC-EVs improve cardiac function by augmenting vessel growth, while reducing ROS generation, inflammation, and fibrosis in MI heart.
Graphical Abstract

Similar content being viewed by others
Introduction
Ischemic heart disease (IHD) is a leading cause of death worldwide [1]. MI leads to myocardium loss, pathological remodeling, dysfunctional cardiac function, and heart failure [2]. MI is manifested by myocyte loss, which triggers a cascade of immune-inflammatory pathways and cellular processes such as complement activation, the generation of ROS, and the activation of inflammasomes. The recovery begins with the action of inflammatory cells, which replace the necrotic myocardium with granulation tissue. Also, fibroblasts generate a new collagen matrix, which eventually results in the formation of the post-infarction scar in the infarcted area [3,4,5].
Recent studies have demonstrated that mesenchymal stem cells (MSCs) can reduce inflammation, fibrotic, and injury to the parenchyma, thus contributing to tissue repair [6,7,8,9,10,11,12]. Earlier clinical studies have been shown that transplanted stem cells can promote tissue regeneration via engraftment in the myocardium, not via differentiation into cardiomyocytes [13, 14]. Despite the benefits, MSCs have not translated well into clinical practice because clinical trials have consistently failed to produce conclusive outcomes possibly due to the difficulties in preparing large scale of homogenous cells that can be applicable for therapeutical purposes [15]. In this regard, MSC-like cells derived from pluripotent stem cells (iMSCs) have tremendous advantages owing to their homogeneity and stable growth profile [16, 17]. These properties make it possible to generate a large quantity of clonally derived iMSCs in a scalable manner [18]. Several animal studies using iMSCs have shown significant benefits in tissue regeneration and repair [19,20,69] suggested that HA-iMSC-EVs share pharmacological functions with several drugs associated with cardiovascular diseases/development. HA is an unsulfated glycosaminoglycan with excellent viscoelasticity, high moisture retention capacity, biocompatibility, and hygroscopic properties [70]. Most cells in the body synthesize HA at some point in their cell cycle, implicating it in several fundamental biological processes. Wang et al. reported that HA oligosaccharides improve angiogenesis by upregulating VEGF secretion and myocardial function reconstruction after MI through the polarization of M2-type macrophages [71]. Le et al. also reported that HA-based microrods provide local biochemical and biomechanical signals to reprogram fibroblasts and attenuate cardiac fibrosis [72]. Recent study also reported that Hapln1-expressing epicardial cells were responsible for the processing and organization of HA within the ECM, which has recently been implicated in heart regeneration [73]. Furthermore, HA priming increased the trafficking, adhesion, and internalization of MSC EV into injured target cells, enhancing the therapeutic potency of the EV. HA may act as a bridge between MSC EV and target cells, allowing the EV to be internalized [37]. Together with these previous findings and the results from the present study, HA-iMSC-EVs have potential as a novel cell-free therapeutic option for MI.
Conventionally, most preclinical/clinical investigations on cell therapy for heart diseases have used single-dose delivery, mostly due to the technical difficulties of repeated administration and high lethality [74]. However, EVs lack some of the risks associated with cell-based therapies due to their low immunogenicity, minimal embolism risk, and biocompatibility. Furthermore, EVs can be delivered to the heart via various delivery routes, including intravenous, intracoronary and intramyocardial administration [75]. In the present study, we compared the therapeutic effects of HA-iMSC-EVs administered via various regime (i.e., delivery routes, dosages, and schedules). TTC/Evan’s blue staining showed that the infarct size was significantly reduced by HA-iMSC-EVs compared to that in the vehicle-treated animals, regardless of the delivery route. However, bioluminescence imaging revealed that most of the intravenously injected HA-iMSC-EVs were localized in off-target organs, including the brain, lungs, liver, and kidneys. Therefore, we decided to administer HA-iMSC-EVs via intramyocardial route to reduce the localization and possible side effects in non-cardiac tissues. We also tried to identify whether the repeated injection of HA-iMSC-EVs, considering clinically feasible platforms, could improve cardiac functions in comparison with single injection. However, we found that the repeated injection of HA-iMSC-EVs did not significantly improve systolic and diastolic cardiac functions. In histologic analysis, only capillary densities in the border zone and percent fibrosis of LV wall were significantly improved in the high (20 mg/kg) dose of repeated injection of HA-iMSC-EVs. Considering that the repeated injection of 10 mg/kg of HA-iMSC-EVs didn’t improve capillary densities and percent fibrosis compared with those of single injection of 20 mg/kg of HA-iMSC-EVs, we can infer that (1) the therapeutic concentration of 20 mg/kg in single injection group was set too higher than expected, and (2) the time point of secondary injection (at day 7 after the first one) may not be sufficient to reverse the inflammation and fibrosis, resulting in reduced dose-dependency in some functional parameters. Thus, we are planning to conduct further studies to investigate optimal applications such as HA-iMSC-EVs concentration and dose intervals, in order to enhance the HA-iMSC-EVs treatment regime.
Conclusion
HA-iMSC-EVs improve cardiac repair via multiple cellular mechanisms including promoting capillary growth and attenuating tissue necrosis after MI. This strategy has potential to become an alternative option for cell-free therapeutics for cardiac repair.
Availability of data and materials
The materials can be provided upon request via email to the corresponding author.
Abbreviations
- AAR:
-
Area at risk
- ADSC:
-
Adipose-derived stem cells
- ATC:
-
Anatomical therapeutic chemical
- ATP:
-
Adenosine triphosphate
- CCK-8:
-
Cell counting cell kit 8
- cTnT:
-
Cardiac troponin T
- DAMP:
-
Danger-associated molecular patterns
- ECM:
-
Extracellular matrix
- EDPVR:
-
End-diastolic pressure–volume relationship
- EF:
-
Ejection fraction
- ESPVR:
-
End-systolic pressure–volume relationship
- EVs:
-
Extracellular vesicles
- FS:
-
Fractional shortening
- HA:
-
Hyaluronic acid
- HUVECs:
-
Human umbilical venous endothelial cells
- I/R:
-
Ischemic reperfusion
- IFN-γ:
-
Interferon-gamma
- IHD:
-
Ischemic heart disease
- IL-13:
-
Interleukin 13
- IL-1β:
-
Interleukin-1 beta
- IL-4:
-
Interleukin 4
- IM:
-
Intramyocardial
- iMSCs:
-
Induced mesenchymal stem cells
- iPSC-CM:
-
Cardiomyocytes derived from iPSC
- IV:
-
Intravenously
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipopolysaccharides
- LV:
-
Left ventricular
- LVEDD:
-
Left ventricular end diastolic diameter
- LVEDV:
-
Left ventricular end diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- LVESD:
-
Left ventricular end systolic diameter
- LVESV:
-
Left ventricular end systolic volume
- LVIDd:
-
Left ventricular internal diastolic dimension
- LVIDs:
-
Left ventricular internal systolic dimension
- MI:
-
Myocardial infarction
- MSCs:
-
Mesenchymal stem cells
- NLRP3:
-
NACHT, LRR, and PYD domain-containing protein 3
- NRCFs:
-
Neonatal rat cardiac fibroblasts
- NRCMs:
-
Neonatal rat cardiomyocytes
- NTA:
-
Nanoparticle tracing analysis
- PMA:
-
Phorbol 12-myristate 13-acete
- PV:
-
Pressure–volume
- ROS:
-
Reactive oxidative species
- SWT:
-
Septal wall thickness
- TEM:
-
Transmission electronic microscopy
- TGF-β:
-
Transforming growth factor- beta
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- VEGF:
-
Vascular endothelial growth factor
- WJ:
-
Wharton’s jelly
References
Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Q Outcomes. 2019. https://doi.org/10.1161/CIRCOUTCOMES.118.005375.
Saleh M, Ambrose JA. Understanding myocardial infarction. F1000Research. 2018;7:1378.
Boulet J, Mehra MR. Left ventricular reverse remodeling in heart failure: remission to recovery. Struct Heart. 2021;5(5):466–81.
Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res. 2012;94(2):293–303.
Cabac-Pogorevici I, Muk B, Rustamova Y, Kalogeropoulos A, Tzeis S, Vardas P. Ischaemic cardiomyopathy. Pathophysiological insights, diagnostic management and the roles of revascularisation and device treatment. Gaps and dilemmas in the era of advanced technology. Eur J Heart Fail. 2020;22(5):789–99.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35.
Sim W-S, Park B-W, Ban K, Park H-J. In situ preconditioning of human mesenchymal stem cells elicits comprehensive cardiac repair following myocardial infarction. Int J Mol Sci. 2021;22(3):1449.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019. https://doi.org/10.3390/cells8080886.
Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349.
Nguyen PK, Rhee JW, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1(7):831–41.
Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20(5–6):419–27.
Fu Y, Karbaat L, Wu L, Leijten J, Both SK, Karperien M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B Rev. 2017;23(6):515–28.
Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE. 2014;9(5):e96725.
Bolli R, Mitrani RD, Hare JM, Pepine CJ, Perin EC, Willerson JT, Traverse JH, Henry TD, Yang PC, Murphy MP, et al. A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021;23(4):661–74.
Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):a6884.
Sabapathy V, Kumar S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J Cell Mol Med. 2016;20(8):1571–88.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19103119.
Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K, Li Y, Xu RH. Concise review: mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source for therapeutic applications. Stem Cells. 2019;37(5):572–81.
Sheyn D, Ben-David S, Shapiro G, De Mel S, Bez M, Ornelas L, Sahabian A, Sareen D, Da X, Pelled G, et al. Human induced pluripotent stem cells differentiate into functional mesenchymal stem cells and repair bone defects. Stem Cells Transl Med. 2016;5(11):1447–60.
Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, Dow S. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7(6):456–67.
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, **a JC, Lai WH, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23.
Wei H, Tan G, Qiu Manasi S, Kong G, Yong P, Koh C, Ooi TH, Lim SY, Wong P, et al. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012;9(2):87–100.
Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020. https://doi.org/10.3390/cells9030660.
Zhang B, Tian X, Hao J, Xu G, Zhang W. Mesenchymal stem cell-derived extracellular vesicles in tissue regeneration. Cell Transplant. 2020;29:096368972090850.
Wang AYL. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22041769.
Yáñez-Mó M, Siljander PRM, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066.
Showalter MR, Wancewicz B, Fiehn O, Archard JA, Clayton S, Wagner J, Deng P, Halmai J, Fink KD, Bauer G, et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem Biophys Res Commun. 2019;512(4):729–35.
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48(3):504–11.
Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Investig. 2017;127(5):1600–12.
Bonafè F, Govoni M, Giordano E, Caldarera CM, Guarnieri C, Muscari C. Hyaluronan and cardiac regeneration. J Biomed Sci. 2014. https://doi.org/10.1186/s12929-014-0100-4.
Yoon SJ, Fang YH, Lim CH, Kim BS, Son HS, Park Y, Sun K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91(1):163–71.
Yoon SJ, Hong S, Fang YH, Song M, Son KH, Son HS, Kim SK, Sun K, Park Y. Differential regeneration of myocardial infarction depending on the progression of disease and the composition of biomimetic hydrogel. J Biosci Bioeng. 2014;118(4):461–8.
Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399–410.
Kim J, Lee SK, Jeong SY, Cho HJ, Park J, Kim TM, Kim S. Cargo proteins in extracellular vesicles: potential for novel therapeutics in non-alcoholic steatohepatitis. J Nanobiotechnology. 2021;19(1):372.
Kavanagh DP, Robinson J, Kalia N. Mesenchymal stem cell priming: fine-tuning adhesion and function. Stem Cell Rev Rep. 2014;10(4):587–99.
López-Ruiz E, Jiménez G, de Álvarez Cienfuegos L, Antic C, Sabata R, Marchal JA, Gálvez-Martín P. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur Cell Mater. 2019;37:186–213.
Zhou L, Hao Q, Sugita S, Naito Y, He H, Yeh C-C, Lee J-W. Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia. Stem Cell Res Ther. 2021. https://doi.org/10.1186/s13287-021-02329-2.
Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274(3):R577-595.
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96(8):881–9.
Altara R, Mallat Z, Booz GW, Zouein FA. The CXCL10/CXCR3 axis and cardiac inflammation: implications for immunotherapy to treat infectious and noninfectious diseases of the heart. J Immunol Res. 2016;2016:4396368.
Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112(3):33.
Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28.
Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab. 2000;71(1–2):418–35.
Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–306.
Liu W, Zhang X, Zhao M, Zhang X, Chi J, Liu Y, Lin F, Fu Y, Ma D, Yin X. Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: a critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiol Biochem. 2015;35(6):2483–500.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119(1):91–112.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-09234-6.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Zhao C, Ikeya M. Generation and applications of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Int. 2018;2018:9601623.
Seki T, Fukuda K. Methods of induced pluripotent stem cells for clinical application. World J Stem Cells. 2015;7(1):116–25.
Forraz N, McGuckin CP. The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011;44:60–9.
Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14(6):11692–712.
Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7(1):1–16.
El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20(5):523–44.
Lee HR, Kim S, Shin S, Jeong SY, Lee DW, Lim SU, Kang JY, Son MY, Lee C, Yu KR, et al. iPSC-derived MSCs are a distinct entity of MSCs with higher therapeutic potential than their donor-matched parental MSCs. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms24010881.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.
Kim S, Kim TM. Generation of mesenchymal stem-like cells for producing extracellular vesicles. World J Stem Cells. 2019;11(5):270–80.
Noronha NC, Mizukami A, Caliari-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131.
Liu GY, Liu Y, Lu Y, Qin YR, Di GH, Lei YH, Liu HX, Li YQ, Wu C, Hu XW, et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol. 2016;13(3):369–78.
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells and Development. 2017;26(9):617–31.
Najar M. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res. 2018;67(6):467–77.
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–77.
Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–43.
Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, **ong Y, Chen G, Qian H, ** C, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353–67.
Davis GE, Senger DR. Endothelial extracellular matrix. Circ Res. 2005;97(11):1093–107.
Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005;9(4):777–94.
Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9(1):33–44.
Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, Gould J, Davis JF, Tubelli AA, Asiedu JK, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. 2017;171(6):1437–52.
Park JG, Mok JS, Han YI, Park TS, Kang KW, Choi CS, Park HD, Park J. Connectivity map** of angiotensin-PPAR interactions involved in the amelioration of non-alcoholic steatohepatitis by Telmisartan. Sci Rep. 2019;9(1):4003.
Yasin A, Ren Y, Li J, Sheng Y, Cao C, Zhang K. Advances in hyaluronic acid for biomedical applications. Front Bioeng Biotechnol. 2022;10:910290.
Wang N, Liu C, Wang X, He T, Li L, Liang X, Wang L, Song L, Wei Y, Wu Q, et al. Hyaluronic acid oligosaccharides improve myocardial function reconstruction and angiogenesis against myocardial infarction by regulation of macrophages. Theranostics. 2019;9(7):1980–92.
Le LV, Mohindra P, Fang Q, Sievers RE, Mkrtschjan MA, Solis C, Safranek CW, Russell B, Lee RJ, Desai TA. Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials. 2018;169:11–21.
Sun J, Peterson EA, Wang AZ, Ou J, Smith KE, Poss KD, Wang J. hapln1 Defines an epicardial cell subpopulation required for cardiomyocyte expansion during heart morphogenesis and regeneration. Circulation. 2022;146(1):48–63.
Wysoczynki M, Khan A, Bolli R. New paradigms in cell therapy. Circ Res. 2018;123(2):138–58.
Mentkowski KI, Snitzer JD, Rusnak S, Lang JK. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018;20(3):50.
Acknowledgements
The experimental scheme and process were created with BioRender.com.
Funding
This research was supported by the Korean Fund for Regenerative Medicine funded by the Ministry of Science and ICT, the Ministry of Health and Welfare (21C0708L1-13, Republic of Korea), and a National Research Foundation of Korea Grant funded by the Korean Government (NRF-2022R1A2C2009067, Republic of Korea).
Author information
Authors and Affiliations
Contributions
SYJ performed in vitro experiments and wrote the manuscript. BWP performed in vivo experiments and wrote the manuscript. JK performed in vitro experiments. SL, HY and JL performed analysis and prepared the experimental materials. SL, JHP, JK, WS, and KB performed in vivo experiment. JP analyzed the bioinformatics data and wrote the manuscript. HJP and SK supervised the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of The Catholic University of Korea (Approval date: 25 June 2021; Approval number: CUMC-2020–0063-03).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig S1.
Characterization of hiPSC-CM. a Characterization of hiPSC-CM was performed by flow cytometric analysis with cardiomyocyte markers including cardiac troponin T (cTnT), actinin alpha (α-actinin), alpha smooth muscle actin (α-SMA) and myosin light chain 2a (MLC2a). Fig S2. Protection effects of the HA-iMSC-EVs on primary neonatal rat cardiomyocyte. a-b EVs were treated to primary neonatal rat cardiomyocyte damaged with 500 μM of H2O2 for 2h. a Video were recorded for 15 seconds, and the beating area were marked yellow line. b After 48 h, relative viable cells were increased in EV-treated groups compared to PBS group. Mean ± SD, n= 3, **p < 0.01 vs PBS; ##p < 0.01 vs iMSC-EV. Fig S3. Heart rate during the cardiac function measurements. During cardiac ultrasound measurements, imaging was conducted on a temperature-controlled pad set at 40 degrees Celsius. Anesthesia was carefully administered using masks. Ultrasound system recorded M-mode images over a 3-second duration. Subsequently, heart rate calculations were derived from M-mode images captured at the 5-week time point. Mean ± SEM, n= 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeong, SY., Park, BW., Kim, J. et al. Hyaluronic acid stimulation of stem cells for cardiac repair: a cell-free strategy for myocardial infarct. J Nanobiotechnol 22, 149 (2024). https://doi.org/10.1186/s12951-024-02410-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02410-x