Abstract
Radiation-induced skin injury (RISI) is a common complication of radiotherapy. Interferon-alpha inducible protein 6 (IFI6) significantly reduces the radiation sensitivity of HaCaT cells. Sodium alginate (SA) has substantial moisturizing properties. Graphene oxide (GO) is a suitable substrate with physical antibacterial properties. Therefore, we designed materials to modify IFI6 using the biogule of polydopamine (PDA) connected to GO/SA. The structure, size, morphology, and elemental compositions of IFI6-PDA@GO/SA were analyzed. Cytological studies suggested that IFI6-PDA@GO/SA is non-toxic to HaCaT cells, with antibacterial properties. It promotes migration and vascularization and inhibits apoptosis. These cells express IFI6 after irradiation. The mouse model suggested that IFI6-PDA@GO/SA promotes wound healing and reduces reactive oxygen species expression. IFI6-PDA@GO/SA accelerates RISI healing, possibly by initiating the SSBP1/HSF1 signaling pathway. In addition, IFI6-PDA@GO/SA improves the immune microenvironment. This study constitutes the first use of IFI6 as a RISI wound-healing material.
Similar content being viewed by others
Introduction
Radiation resembles the standard treatment protocol for cancer types such as head and neck, breast, and lung cancers [1]. However, many complications are associated with radiotherapy reactions, limiting the radiotherapy dose and treatment effect. Radiation-induced skin injury (RISI) is a common but debilitating side effect; various degrees of radiodermatitis occur in 95% of cancer patients following radiotherapy [2B, F). C, N, and O were identified in IFI6, C and O presented in SA, and Na, CI, and Ca appeared in PDA@GO. These findings suggest that IFI6, SA, and PDA@GO were found in the composite sponge. GO sheets were obtained using a modified Hummer’s method with an average size of 2 μm measured using Image J software and a thickness of about 1.5 nm measured by Atomic Force Microscope [18]. To characterize the chemical composition of the Fe3O4NPs-GO-PDA composite thin film, Jang et al. suggested that the C peak (284.08 eV), the peak of C−C (284.08 eV), and the peak of O 1s (531.95 eV) were remarkably dominant, suggesting that the composite system consisted of GO sheets; C−O and C−N binding energies were observed at 286.56 and 285.79 eV, respectively, suggesting that the PDA contains C−N bonds [19].
Liu et al. found characteristic absorption bands of GO sheets at 1054 cm−1 (alkoxy), 1224 cm−1 (epoxy), 1401 cm−1 (carboxyl; C–O), and 1724 cm−1 (carboxyl; C=O) [18]. The new adsorption peak at 1579 cm−1 of the MPDA@GO composites may be attributed to the deformation vibration of N–H bonding and the stretching vibration of C–N bonding. The FTIR results in this paper are consistent with these findings (Fig. 2D), suggesting that the reaction may have occurred between epoxide groups of GO and amine groups of PDA during the preparation of PDA@GO composites. There are no reports of the characteristic absorption peak of IFI6. However, we believe that the 3400 cm−1 position may be associated with the composition of IFI6.
To further test the loading of IFI6, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis was conducted (Fig. 2C). We found that the IFI6- PDA@GO/SA material expressed IFI6, consistent with the IFI6 protein bands as reported [13], suggesting that IFI6 was successfully tested. IFI6-PDA@GO/SA displays a typical nanostructure, and the diameter is about 162 nm, PDI = 0.19, and zeta potential = − 14.64 mV (Fig. 2G). The ultraviolet spectrum of IFI6-PDA@GO/SA showed no evident change in 400–800 nm (Fig. 2E). Zhao et al. synthesized polyelectrolyte complex nanoparticles for plasmid delivery [20]. Nanosized SA solids ranged from 0.04% to 0.08%, and higher concentrations resulted in larger agglomerate-like solution systems. Active targeting of small particles has advantages over passive targeting of large nanospheres due to the enhanced permeability and retention effect. ** novel hydrogel wound dressings; to this end, various antibacterial agents have been introduced into the hydrogels. Huang et al. reported that the hydrogels containing PDA@Ag5GO1 (Ag5GO1 denotes that the mass ratio between Ag and GO is 5:1) exhibited effective antibacterial properties and high inhibition of E. coli and S. aureus [23]. Liu et al. showed that CNF hydrogels and MPDA@GO (1:2)/CNF composite hydrogels showed no antibacterial effect against E. coli and S. aureus [18]. The (MPDA-TH)@GO (1:2)/CNF composite hydrogel inhibited E. coli growth with a zone width of 15 mm, whereas the drug-loaded composite hydrogel exhibited a zone width of 18 mm against S. aureus. This finding suggests that the inhibition zone of the (MPDA-TH)@GO (1:2)/CNF composite hydrogel depends on the released TH from the composite hydrogel. MRSA (G+) and E. coli (G−) in the control group grew well; however, PDA@GO inhibited growth (Fig. 3B, D). PDA@GO /SA inhibited bacterial growth to a greater extent, and IFI6- PDA@GO /SA did not show sufficient bacteriostasis. These findings may be related to the fact that IFI6 has no reported antibacterial activity, while GO and SA have been reported to have good antibacterial activity.
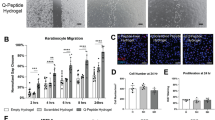
In vitro cytological study of IFI6-PDA@GO/SA
We performed flow cytometry assays to determine the potential role of IFI6 in apoptosis. IFI6 is only expressed in higher eukaryotes [12]. Radiation significantly increased the apoptosis rate (Fig. 4A, C). Compared with Group B, Group E had a lower apoptosis rate (P < 0.05), and the effect in Group E was higher than that of Group D. This finding suggests that IFI6-PDA@GO/SA significantly reduces apoptosis and that the increased IFI6 protein in Group E participates in regulating apoptosis. These phenomena may be related to anti-ROS mechanisms.
IFI6 overexpression resulted in decreased apoptosis in irradiated HaCaT and WS1 cells compared with control skin cells, suggesting a survival-promoting function of IFI6. The effect of IFI6 on mitochondrial membrane potential was investigated (an essential indicator of mitochondrial function) using JC-1 staining [13]. After irradiation, the authors showed that IFI6-silenced WS1 cells had decreased mitochondrial membrane potential. IFI6 regulated melanoma development and growth through E2F2-mediated DNA replication [24] and promoted breast cancer cell metastasis by inducing deregulation of the mitochondrial redox state [25]. In the present study, we showed that overexpression of IFI6 in HaCaT cells promoted cell survival through anti-apoptosis and guided local aggregation of surviving cells by promoting cell migration, suggesting a new function of IFI6 promoting tumor cell survival. Yin et al. found that overexpression of CTD-3252C9.4 facilitated apoptosis of pancreatic cancer cells in vitro and in vivo [7), suggesting a potential interaction between IFI6 and SSBP1 under radiation induction. In the heat shock response (HSR), SSBP 1 relocates to the nucleus by interacting with heat shock transcription factor 1 (HSF1) [36]. Jia et al. proposed that IFI6 is involved in HSF1 mediated HSR [13]. As expected, radiation enhanced HSF1 transcriptional activity was blocked in IFI6 knockout cells but enhanced in IFI6-overexpressing cells. HSF1-mediated HSR is a typical anti-toxic stress response, including heat shock and oxidative stress.
As shown in the immunohistochemistry studies, Group A showed less expression of IFI6 and its downstream pathway SSBP1/HSF1 protein, and the positive expression of related proteins (Group B) increased significantly after 30 Gy radiation (Fig. 7A, B). IFI6-PDA@GO/SA (Group E) had the most potent effect of promoting protein expression (P < 0.05). As shown in Fig. 7C, D, the IFI6 protein in wound tissue significantly increased, suggesting that IFI6-PDA@GO/SA material released IFI6 into the wound. IFI6 co-localizes with SSBP1 to initiate the expression of HSF1, thereby mediating the downstream HSR. Elevated expression of heat shock proteins regulated by HSF1 is radioprotective for tumor cells [36]. HSF1 targets genes such as ppl to interact with AKT1, a kinase that mediates a variety of cell growth and survival signal transduction processes [13]. These states illustrate the complex mechanisms by which IFI6 regulates the radiation sensitivity of human skin cells.
The effect of IFI6-PDA@GO/SA on the immune microenvironment
Immune cells, particularly regulatory T (Treg) cells, play a critical role in wound healing. When wound formation and inflammatory responses occur, immune cells help clear foreign antigens [37]. Treg cells suppress the activation of the immune system and prevent pathological self-reactivity such as autoimmune diseases. Cytokines participate in cell–cell interactions and communication and are involved in cell migration, proliferation, and inflammatory responses. Kim et al. found that CD4+ and CD8+ Treg cells in cm had a variety of cytokines and growth factors [38]. CD4+ and CD8+ Treg cells stimulated HaCaT keratinocyte migration through EMT and upregulation of MMP-1.
To determine whether the IFI6-PDA@GO/SA regulates the immune microenvironment within the wound and explore the mechanism, wound-draining lymph nodes were collected 14 days after irradiation. Flow cytometry showed that Group E had significant activation of CD4+ and CD8 +T cells (Fig. 8A, B). These findings suggest that Treg cells which contains various cytokines and growth factors, stimulates cell migration and proliferation to promote wound healing. IFI6-PDA@GO/SA also promoted the infiltration of NK and M1 cells (Fig. 8C, D). Sobecki et al. demonstrated that the lack of hypoxia-inducible factor (HIF)-1α in NK cell α hypomorphic mice exhibit the cytokine interferon-γ and impaired release of granulocyte–macrophage colony-stimulating factor as part of a blunted immune response [39]. HIF-1 in NK cells α is the link that balances antimicrobial skin defenses and overall repair.
It is worth highlighting that we found that IFI6-PDA@GO/SA elevated NK cells more significantly than CD4+ and CD8+ cells. On the other hand, IFI6-PDA@GO/SA promoted CD4+ and CD8+ expression in wound cells, thereby increasing T cell activation and NK cell infiltration, realizing the synergistic effect of reducing sensitization in RISI.
Conclusions
We designed and fabricated a nanomaterial combining IFI6 for RISI wound healing. Based on our overall evaluation of IFI6-PDA@GO/SA nanomaterials, we conclude that GO possesses excellent antibacterial activity, infrastructure, and biocompatibility. PDA possesses excellent anti-ROS and bioadhesion properties. SA possesses excellent antibacterial activity and moisture retention. The most important finding is that IFI6 promotes cell proliferation, migration, and vascularization and stimulates the immune microenvironment. The five components work together to promote RISI healing. IFI6 acts synergistically to reduce oxidative stress and inflammation by activating the SSBP1/HSF1 signaling pathway.
IFI6-PDA@GO/SA improves inflammation in RISI wounds and induces granulation tissue formation, angiogenesis, and collagen deposition, resulting in faster wound closure. We believe this nanomaterial provides a valuable alternative and promising strategy for RISI wound repair.
References
Sörgel CA, Schmid R, Stadelmann N, Weisbach V, Distel L, Horch RE, Kengelbach-Weigand A. IGF-I and hyaluronic acid mitigate the negative effect of irradiation on human skin keratinocytes. Cancers. 2022;14:588.
Zhao M, Wang C, **e J, Ji C, Gu Z. Eco-friendly and scalable synthesis of fullerenols with high free radical scavenging ability for skin radioprotection. Small. 2021;17:2102035.
Cy H. TF T: use of H-1 antihistamine in dermatology: more than itch and urticaria control: a systematic review. Dermatol Ther. 2021;11:719–32.
**e J, Zhao M, Wang C, Yong Y, Gu Z, Zhao Y. Rational design of nanomaterials for various radiation-induced diseases prevention and treatment. Adv Healthc Mater. 2021;10:2001615.
Zhang L, Yu Y, Zheng S, Zhong L, Xue J. Preparation and properties of conductive bacterial cellulose-based graphene oxide-silver nanoparticles antibacterial dressing. Carbohyd Polym. 2021;257: 117671.
Fu C, Jiang Y, Yang X, Wang Y, Ji W, Jia G. Mussel-inspired gold nanoparticle and PLGA/L-lysine-g-graphene oxide composite Scaffolds for bone defect repair. Int J Nanomed. 2021;16:6693–718.
Zhang Y, Bian T, Jiang R, Zhang Y, Zheng X, Li Z. Bionic chitosan-carbon imprinted aerogel for high selective recovery of Gd(III) from end-of-life rare earth productions. J Hazard Mater. 2021;407: 124347.
Weng Z, Yu F, Leng Q, Zhao S, Xu Y, Zhang W, Zhu Z, Ye J, Wei Q, Wang X. Electrical and visible light dual-responsive ZnO nanocomposite with multiple wound healing capability. Mater Sci Eng C. 2021;124: 112066.
Zhang L, Yu Y, Zheng S, Zhong L, Xue J. Preparation and properties of conductive bacterial cellulose-based graphene oxide-silver nanoparticles antibacterial dressing. Carbohydr Polym. 2021;257: 117671.
**n Y, **gyan Y, **tian C, Ruiqi N, Yanhao Z, Hao S, Liang J, Tingting T, Yi P. LncRNA CTD-3252C9.4 modulates pancreatic cancer cell survival and apoptosis through regulating IFI6 transcription. Cancer Cell Int. 2021;21:433.
Muhamma S, Hafiz U, Kun Y, Miao H, Jianpeng F, MuhammadAdman S, Ruidong H, Qiaohong L, Deyin G, Yu C, Li Z. The functional and antiviral activity of interferon alpha-inducible IFI6 against hepatitis B virus replication and gene expression. Front Immunol. 2021;12:634937.
Lin X, Tingjian Z, Tao L, Min L, Jun M, Fuxiang B, Guanyi L, Jie W, Hui L, Cord B, et al. ATF3 downmodulates its new targets IFI6 and IFI27 to suppress the growth and migration of tongue squamous cell carcinoma cells. Plos Genet. 2021;17:e1009283.
Jia H, Mo W, Hong M, Jiang S, Zhang Y, He D, Yu D, Shi Y, Cao J, Xu X, Zhang S. Interferon-α inducible protein 6 (IFI6) confers protection against ionizing radiation in skin cells. J Dermatol Sci. 2020;100:139–47.
Solovieva EV, Teterina AY, Klein OI, Komlev VS, Alekseev AA, Panteleyev AA. Sodium alginate-based composites as a collagen substitute for skin bioengineering. Biomed Mater. 2020;16:15002.
Ma S, Wu S, Zhang J, Song Y, Tang H, Zhang K, Huang F, Cao Y. Heptacyclic S, N-heteroacene-based near-infrared nonfullerene acceptor enables high-performance organic solar cells with small highest occupied molecular orbital offsets. ACS Appl Mater Interfaces. 2020;12:51776–84.
Zhu Y, Ma Z, Kong L, He Y, Chan HF, Li H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials. 2020;256: 120216.
Ma W, Ma H, Qiu P, Zhang H, Yang Z, Ma B, Chang J, Shi X, Wu C. Sprayable β-FeSi2 composite hydrogel for portable skin tumor treatment and wound healing. Biomaterials. 2021;279: 121225.
Liu Y, Fan Q, Huo Y, Liu C, Li B, Li Y. Construction of a mesoporous Polydopamine@GO/cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. Acs Appl Mater Inter. 2020;12:57410–20.
Jang J, Song SH, Kim H, Moon J, Ahn H, Jo K, Bang J, Kim H, Koo J. Janus graphene oxide sheets with Fe3O4 nanoparticles and polydopamine as anodes for lithium-ion batteries. Acs Appl Mater Inter. 2021;13:14786–95.
Zhao R, ** X, Li A, Xu B, Shen Y, Wang W, Huang J, Zhang Y, Li X. Precise diabetic wound therapy: PLS nanospheres eliminate senescent cells via DPP4 targeting and PARP1 activation. Adv Sci. 2021;9:2104128.
**e H, **a H, Huang L, Zhong Z, Ye Q, Zhang L, Lu A. Biocompatible, antibacterial and anti-inflammatory zinc ion cross-linked quaternized cellulose-sodium alginate composite sponges for accelerated wound healing. Int J Biol Macromol. 2021;191:27–39.
Lu H, Xu S, Guo Z, Zhao M, Liu Z. Redox-responsive molecularly imprinted nanoparticles for targeted intracellular delivery of protein toward cancer therapy. ACS Nano. 2021;15:18214–25.
Huang H, He D, Liao X, Zeng H, Fan Z. An excellent antibacterial and high self-adhesive hydrogel can promote wound fully healing driven by its shrinkage under NIR. Mater Sci Eng, C. 2021;129: 112395.
Gupta R, Forloni M, Bisserier M, Dogra SK, Yang Q, Wajapeyee N. Interferon alpha-inducible protein 6 regulates NRASQ61K-induced melanomagenesis and growth. Elife. 2016;5.
Cheriyath V, Kaur J, Davenport A, Khalel A, Chowdhury N, Gaddipati L. G1P3 (IFI6), a mitochondrial localised antiapoptotic protein, promotes metastatic potential of breast cancer cells through mtROS. Br J Cancer. 2018;119:52–64.
Liu Z, Gu S, Lu T, Wu K, Li L, Dong C, Zhou Y. IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress. J Exp Clin Canc Res. 2020;39:144.
Kinoda J, Ishihara M, Nakamura S, Fujita M, Fukuda K, Sato Y, Yokoe H. Protective effect of FGF-2 and low-molecular-weight heparin/protamine nanoparticles on radiation-induced healing-impaired wound repair in rats. J Radiat Res. 2018;59:27–34.
Kyritsi A, Kikionis S, Tagka A, Koliarakis N, Evangelatou A, Papagiannis P, Stratigos A, Karalis V, Dallas P, Vitsos A, et al. Management of acute radiodermatitis in non-melanoma skin cancer patients using electrospun nanofibrous patches loaded with Pinus halepensis bark extract. Cancers. 2021;13:2596.
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Li HM, Zhang WS, Chen CY, **e H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–84.
Ahmad I, Muneer KM, Chang ME, Nasr HM, Clay JM, Huang CC, Yusuf N. Ultraviolet radiation-induced downregulation of SERCA2 mediates activation of NLRP3 inflammasome in basal cell carcinoma. Photochem Photobiol. 2017;93:1025–33.
Wei J, Wang H, Wang H, Wang B, Meng L, **n Y, Jiang X. The role of NLRP3 inflammasome activation in radiation damage. Biomed Pharmacother. 2019;118: 109217.
Hasegawa T, Nakashima M, Suzuki Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem Biophys Res Commun. 2016;477:329–35.
Srinivas US, Tan B, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25: 101084.
Yang X, Ren H, Guo X, Hu C, Fu J. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Albany NY). 2020;12:23379–93.
Renaudin X. Reactive oxygen species and DNA damage response in cancer. Int Rev Cell Mol Biol. 2021;364:139–61.
Miralles FJ, Shi Y, Wanrooij S, Zhu X, Jemt E, Persson O, Sabouri N, Gustafsson CM, Falkenberg M. In vivo occupancy of mitochondrial single-stranded DNA binding protein supports the strand displacement mode of DNA replication. Plos Genet. 2014;10: e1004832.
Prakoso YA, Hidayah N, Rini CS, Kurniasih K. Dynamic change of blood profile in rat models with acute skin injury artificially infected with methicillin-resistant Staphylococcus aureus. Vet World. 2021; 2085–2090.
Kim D, Lo E, Kim D, Kang J. Regulatory T cells conditioned media stimulates migration in HaCaT keratinocytes: involvement of wound healing. Clin Cosmet Investig Dermatol. 2020;13:443–53.
Sobecki M, Krzywinska E, Nagarajan S, Audigé A, Huỳnh K, Zacharjasz J, Debbache J, Kerdiles Y, Gotthardt D, Takeda N, et al. NK cells in hypoxic skin mediate a trade-off between wound healing and antibacterial defence. Nat Commun. 2021;12.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81672856), the Military Program of Army Medical University (CX2019JS104; 2021XJS17). The General Program of Chongqing Natural Science Foundation (cstc2021jcyj-msxmX0687), the Medical and Industrial Combination Cultivation Program of Southwest Jiaotong University (2682021ZTPY019) and the Spark Program of General Hospital of Western Theater Command of PLA.
Author information
Authors and Affiliations
Contributions
JH: Writing—review and editing. MS: Conceptualization, Methodology. DL: Validation, Formal analysis. TZ: Investigation. JL: Conceptualization, Methodology, Writing—review and editing, Supervision, Project administration, Funding acquisition. DZ: Writing—original draft, Writing—review and editing, Visualization. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hao, J., Sun, M., Li, D. et al. An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of the HSF1 activity. J Nanobiotechnol 20, 288 (2022). https://doi.org/10.1186/s12951-022-01466-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01466-x