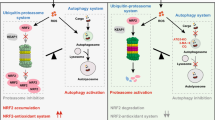
Abstract
Copper oxide nanoparticles (CuONPs) are widely used metal oxide NPs owing to their excellent physical–chemical properties. Circulation translocation of CuONPs after inhalation leads to vascular endothelial injury. Mitochondria, an important regulatory hub for maintaining cell functions, are signaling organelles in responses to NPs-induced injury. However, how mitochondrial dynamics (fission and fusion) and mitophagy (an autophagy process to degrade damaged mitochondria) are elaborately orchestrated to maintain mitochondrial homeostasis in CuONPs-induced vascular endothelial injury is still unclear. In this study, we demonstrated that CuONPs exposure disturbed mitochondrial dynamics through oxidative stress-dependent manner in vascular endothelial cells, as evidenced by the increase of mitochondrial fission and the accumulation of fragmented mitochondria. Inhibition of mitochondrial fission with Mdivi-1 aggravated CuONPs-induced mtROS production and cell death. Furthermore, we found that mitochondrial fission led to the activation of PINK1-mediated mitophagy, and pharmacological inhibition with wortmannin, chloroquine or genetical inhibition with siRNA-mediated knockdown of PINK1 profoundly repressed mitophagy, suggesting that the protective role of mitochondrial fission and PINK1-mediated mitophagy in CuONPs-induced toxicity. Intriguingly, we identified that TAX1BP1 was the primary receptor to link the ubiquitinated mitochondria with autophagosomes, since TAX1BP1 knockdown elevated mtROS production, decreased mitochondrial clearance and aggravated CuONPs-induced cells death. More importantly, we verified that urolithin A, a mitophagy activator, promoted mtROS clearance and the removal of damaged mitochondria induced by CuONPs exposure both in vitro and in vivo. Overall, our findings indicated that modulating mitophagy may be a therapeutic strategy for pathological vascular endothelial injury caused by NPs exposure.
Graphical Abstract

Highlights
-
1.
CuONPs disturb mitochondrial dynamics and trigger mitophagy in vascular endothelial cells and mouse blood vessel.
-
2.
PINK1/TAX1BP1-mediated mitophagy regulates the removal of excessive ROS and aberrant mitochondria in CuONPs-treated vascular endothelial cells.
-
3.
The mitophagy activator urolithin A attenuates CuONPs-induced vascular endothelial cells death and mice vascular injury.
Similar content being viewed by others
Introduction
Copper oxide nanoparticles (CuONPs) are widely used in industrial and biomedical fields owing to their excellent physical–chemical properties, earth-abundant and inexpensive properties [1]. For example, CuONPs have been efficiently used for biological sensors to detect glucose, cholesterol, disease-related protein biomarkers and others [2]. CuONPs nanomedicines have been used against various types of tumors [3,4,5]. In addition, CuONPs show excellent antimicrobial activities against Staphylococcus aureus and Escherichia coli [6]. Currently, CuONPs is also developed as antiviral surface coatings suppressing the spread of SARS-CoV-2 [7,8,9]. Meanwhile, CuO nanocomposites are designed as a potential treatment against bacterial infected diabetic non-healing wounds [10, 11].
However, the widespread application of CuONPs in industrial and biomedical fields has seriously threatened the health of this human being. CuONPs are recognized as particularly highly toxic NPs, when compared to many other metal oxide NPs [1, 12]. Accumulating evidence shows that the transformation of nanomaterials in the environment or living systems is closely related to nanomaterials stability and toxicity [13, 14]. CuONPs also undergo chemical transformation under conditions relevant to living systems and the natural environment. CuONPs dissolve at lysosomal pH (4–5) solution and undergo sulfidation by a dissolution-reprecipitation mechanism [15]. Consistently, in our previous in-vitro study, we confirmed that lysosomal deposition of CuONPs facilitated the release of Cu ions from CuONPs-treated vascular endothelial cells [16]. However, the underlying protective mechanisms against CuONPs toxicity are not yet fully understood.
Inhaled NPs are closely linked to cardiovascular diseases [17,18,19]. Exposure to combustion-derived NPs impairs vascular physiological functions and inhibits arterial vasodilatation both in pre-clinical (rat model) and clinical model [20]. Pulmonary exposure to engineered NPs results in oxidative stress and inflammation, consequently contributes to the progression of atherosclerosis [21,22,23]. Vascular endothelial cells cover the inner surface of all blood vessels and serve as a regulatory hub of systemic circulation [24]. Because inhaled NPs can translocate into systemic circulation and accumulate at the sites of vascular disease, NPs may directly interact with vascular endothelial cells and impair vascular physiological functions [25].
Mitochondrial dynamics is an important metabolic and regulatory hub characterized by mitochondrial fusion, fission and degradation of damaged mitochondria [26]. Disruption of mitochondrial dynamics leads to multiple diseases such as cancers, cardiovascular disease, neurodegenerative disease, aging and others [27]. We previously reported that CuONPs treatment resulted in mitochondrial dysfunction and excessive mitochondrial ROS (mtROS) production in vascular endothelial cells [16, 3.
Statistical analysis
The data were shown as mean ± standard deviation (S.D.). Each experiment was repeated three times. Data were analyzed by two-tailed unpaired Student's t-test for two groups comparisons or one-way ANOVA followed by Tukey's test for comparisons of parameters among multiple groups. All statistical analyses were taken with GraphPad Prism 5.0 software (San Diego, CA, USA). *p < 0.05 was considered statistical significance.
Availability of data and materials
The authors declare that data related to this study are provided upon request.
References
Gawande MB, Goswami A, Felpin FX, Asefa T, Huang X, Silva R, et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev. 2016;116(6):3722–811. https://doi.org/10.1021/acs.chemrev.5b00482.
Verma N, Kumar N. Synthesis and biomedical applications of copper oxide nanoparticles: an expanding horizon. ACS Biomater Sci Eng. 2019;5(3):1170–88. https://doi.org/10.1021/acsbiomaterials.8b01092.
Xu H, Yuan R, Liu X, Li X, Qiao G, Li C, et al. Zn-doped CuO nanocomposites inhibit tumor growth by NF-kappaB pathway cross-linked autophagy and apoptosis. Nanomedicine (Lond). 2019;14(2):131–49. https://doi.org/10.2217/nnm-2018-0366.
Mani VM, Kalaivani S, Sabarathinam S, Vasuki M, Soundari A, Ayyappa Das MP, et al. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: bioactivity and anti-cancer evaluations. Environ Res. 2021;201: 111502. https://doi.org/10.1016/j.envres.2021.111502.
Chen H, Feng X, Gao L, Mickymaray S, Paramasivam A, Abdulaziz Alfaiz F, et al. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif Cells Nanomed Biotechnol. 2021;49(1):240–9. https://doi.org/10.1080/21691401.2021.1890101.
Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009;33(6):587–90. https://doi.org/10.1016/j.ijantimicag.2008.12.004.
Imani SM, Ladouceur L, Marshall T, Maclachlan R, Soleymani L, Didar TF. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14(10):12341–69. https://doi.org/10.1021/acsnano.0c05937.
Tortella GR, Pieretti JC, Rubilar O, Fernandez-Baldo M, Benavides-Mendoza A, Diez MC, et al. Silver, copper and copper oxide nanoparticles in the fight against human viruses: progress and perspectives. Crit Rev Biotechnol. 2021. https://doi.org/10.1080/07388551.2021.1939260.
Merkl P, Long S, McInerney GM, Sotiriou GA. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials (Basel). 2021. https://doi.org/10.3390/nano11051312.
Qiao Y, He J, Chen W, Yu Y, Li W, Du Z, et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–315. https://doi.org/10.1021/acsnano.9b08930.
Sen S, Sarkar K. Effective biocidal and wound healing cogency of biocompatible glutathione: citrate-capped copper oxide nanoparticles against multidrug-resistant pathogenic enterobacteria. Microb Drug Resist. 2021;27(5):616–27. https://doi.org/10.1089/mdr.2020.0131.
Sun T, Yan Y, Zhao Y, Guo F, Jiang C. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS ONE. 2012;7(8):e43442. https://doi.org/10.1371/journal.pone.0043442.
Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environ Sci Technol. 2012;46(13):6893–9. https://doi.org/10.1021/es300839e.
Setyawati MI, Zhao Z, Ng KW. Transformation of nanomaterials and its implications in gut nanotoxicology. Small. 2020;16(36):e2001246. https://doi.org/10.1002/smll.202001246.
Wang Z, von dem Bussche A, Kabadi PK, Kane AB, Hurt RH. Biological and environmental transformations of copper-based nanomaterials. ACS Nano. 2013;7(10):8715–27. https://doi.org/10.1021/nn403080y.
Zhang J, Zou Z, Wang B, Xu G, Wu Q, Zhang Y, et al. Lysosomal deposition of copper oxide nanoparticles triggers HUVEC cells death. Biomaterials. 2018;161:228–39. https://doi.org/10.1016/j.biomaterials.2018.01.048.
Hadrup N, Zhernovkov V, Jacobsen NR, Voss C, Strunz M, Ansari M, et al. Acute Phase response as a biological mechanism-of-action of (nano)particle-induced cardiovascular disease. Small. 2020;16(21):e1907476. https://doi.org/10.1002/smll.201907476.
Liu X, Wei W, Liu Z, Song E, Lou J, Feng L, et al. Serum apolipoprotein A-I depletion is causative to silica nanoparticles-induced cardiovascular damage. Proc Natl Acad Sci U S A. 2021. https://doi.org/10.1073/pnas.2108131118.
Raftis JB, Miller MR. Nanoparticle translocation and multi-organ toxicity: a particularly small problem. Nano Today. 2019;26:8–12. https://doi.org/10.1016/j.nantod.2019.03.010.
Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32(21):2660–71. https://doi.org/10.1093/eurheartj/ehr195.
Kang GS, Gillespie PA, Gunnison A, Moreira AL, Tchou-Wong KM, Chen LC. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect. 2011;119(2):176–81. https://doi.org/10.1289/ehp.1002508.
Lee DK, Jang HS, Chung H, Jeon S, Jeong J, Choi JH, et al. Aggravation of atherosclerosis by pulmonary exposure to indium oxide nanoparticles. Nanotoxicology. 2020;14(3):355–71. https://doi.org/10.1080/17435390.2019.1704590.
Ma R, Qi Y, Zhao X, Li X, Sun X, Niu P, et al. Amorphous silica nanoparticles accelerated atherosclerotic lesion progression in ApoE(-/-) mice through endoplasmic reticulum stress-mediated CD36 up-regulation in macrophage. Part Fibre Toxicol. 2020;17(1):50. https://doi.org/10.1186/s12989-020-00380-0.
Kruger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20184411.
Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11(5):4542–52. https://doi.org/10.1021/acsnano.6b08551.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–59. https://doi.org/10.1146/annurev-pathmechdis-012419-032711.
Russell OM, Gorman GS, Lightowlers RN, Turnbull DM. Mitochondrial diseases: hope for the future. Cell. 2020;181(1):168–88. https://doi.org/10.1016/j.cell.2020.02.051.
He H, **ao S, Xu G, Wang B, Zou Z, Qin X, et al. The NADPH oxidase 4 protects vascular endothelial cells from copper oxide nanoparticles-induced oxidative stress and cell death. Life Sci. 2020;252: 117571. https://doi.org/10.1016/j.lfs.2020.117571.
Zhang J, Wang B, Wang H, He H, Wu Q, Qin X, et al. Disruption of the superoxide anions-mitophagy regulation axis mediates copper oxide nanoparticles-induced vascular endothelial cell death. Free Radic Biol Med. 2018;129:268–78. https://doi.org/10.1016/j.freeradbiomed.2018.09.032.
Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy. 2011;7(3):297–300. https://doi.org/10.4161/auto.7.3.14502.
Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. https://doi.org/10.1038/nrm3028.
Qi Y, Ma R, Li X, Lv S, Liu X, Abulikemu A, et al. Disturbed mitochondrial quality control involved in hepatocytotoxicity induced by silica nanoparticles. Nanoscale. 2020;12(24):13034–45. https://doi.org/10.1039/d0nr01893g.
Li J, Chang X, Shang M, Niu S, Zhang W, Li Y, et al. The crosstalk between DRP1-dependent mitochondrial fission and oxidative stress triggers hepatocyte apoptosis induced by silver nanoparticles. Nanoscale. 2021;13(28):12356–69. https://doi.org/10.1039/d1nr02153b.
Wu D, Lu J, Ma Y, Cao Y, Zhang T. Mitochondrial dynamics and mitophagy involved in MPA-capped CdTe quantum dots-induced toxicity in the human liver carcinoma (HepG2) cell line. Environ Pollut. 2021;274: 115681. https://doi.org/10.1016/j.envpol.2020.115681.
Li J, Chang X, Shang M, Niu S, Zhang W, Zhang B, et al. Mitophagy-lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells. Ecotoxicol Environ Saf. 2021;208: 111463. https://doi.org/10.1016/j.ecoenv.2020.111463.
Wei L, Wang J, Chen A, Liu J, Feng X, Shao L. Involvement of PINK1/parkin-mediated mitophagy in ZnO nanoparticle-induced toxicity in BV-2 cells. Int J Nanomedicine. 2017;12:1891–903. https://doi.org/10.2147/IJN.S129375.
**ao J, Tu B, Zhou X, Jiang X, Xu G, Zhang J, et al. Autophagy deficiency exacerbates acute lung injury induced by copper oxide nanoparticles. J Nanobiotechnology. 2021;19(1):162. https://doi.org/10.1186/s12951-021-00909-1.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105–17. https://doi.org/10.1016/j.tem.2015.12.001.
Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–22. https://doi.org/10.1038/s41556-018-0176-2.
Liu L, Li Y, Wang J, Zhang D, Wu H, Li W, et al. Mitophagy receptor FUNDC1 is regulated by PGC-1alpha/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021;22(3):e50629. https://doi.org/10.15252/embr.202050629.
Takahashi D, Moriyama J, Nakamura T, Miki E, Takahashi E, Sato A, et al. AUTACs: cargo-specific degraders using selective autophagy. Mol Cell. 2019;76(5):797-810 e10. https://doi.org/10.1016/j.molcel.2019.09.009.
Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8(10):1462–76. https://doi.org/10.4161/auto.21211.
Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–31. https://doi.org/10.1038/ncb2012.
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–14. https://doi.org/10.1038/nature14893.
Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc Natl Acad Sci U S A. 2016;113(15):4039–44. https://doi.org/10.1073/pnas.1523926113.
D’Amico D, Andreux PA, Valdes P, Singh A, Rinsch C, Auwerx J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol Med. 2021;27(7):687–99. https://doi.org/10.1016/j.molmed.2021.04.009.
Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–88. https://doi.org/10.1038/nm.4132.
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–12. https://doi.org/10.1038/s41593-018-0332-9.
Lee HJ, Jung YH, Choi GE, Kim JS, Chae CW, Lim JR, et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2021;28(1):184–202. https://doi.org/10.1038/s41418-020-0593-1.
Luan P, D’Amico D, Andreux PA, Laurila PP, Wohlwend M, Li H, et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abb0319.
Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–54. https://doi.org/10.1038/s41556-018-0124-1.
Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17(12):865–86. https://doi.org/10.1038/nrd.2018.174.
Jannesari M, Akhavan O, Madaah Hosseini HR, Bakhshi B. Graphene/CuO2 nanoshuttles with controllable release of oxygen nanobubbles promoting interruption of bacterial respiration. ACS Appl Mater Interfaces. 2020;12(32):35813–25. https://doi.org/10.1021/acsami.0c05732.
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. https://doi.org/10.1126/science.1219855.
Sprenger HG, Langer T. The good and the bad of mitochondrial breakups. Trends Cell Biol. 2019;29(11):888–900. https://doi.org/10.1016/j.tcb.2019.08.003.
Ma W, He S, Ma H, Jiang H, Yan N, Zhu L, et al. Silver nanoparticle exposure causes pulmonary structural damage and mitochondrial dynamic imbalance in the rat: protective effects of sodium selenite. Int J Nanomedicine. 2020;15:633–45. https://doi.org/10.2147/IJN.S232986.
He H, Zou Z, Wang B, Xu G, Chen C, Qin X, et al. Copper oxide nanoparticles induce oxidative DNA damage and cell death via copper ion-mediated P38 MAPK activation in vascular endothelial cells. Int J Nanomedicine. 2020;15:3291–302. https://doi.org/10.2147/IJN.S241157.
Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53(8):1783–94. https://doi.org/10.1007/s00125-010-1770-4.
Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2012;52(2):348–56. https://doi.org/10.1016/j.freeradbiomed.2011.10.491.
Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79(2):341–51. https://doi.org/10.1093/cvr/cvn104.
Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31(4):570–85. https://doi.org/10.1016/j.molcel.2008.08.002.
Moyzis AG, Lally NS, Liang W, Leon LJ, Najor RH, Orogo AM, et al. Mcl-1-mediated mitochondrial fission protects against stress but impairs cardiac adaptation to exercise. J Mol Cell Cardiol. 2020;146:109–20. https://doi.org/10.1016/j.yjmcc.2020.07.009.
Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell. 2017;67(6):922-35 e5. https://doi.org/10.1016/j.molcel.2017.08.013.
Mao K, Klionsky DJ. Mitochondrial fission facilitates mitophagy in Saccharomyces cerevisiae. Autophagy. 2013;9(11):1900–1. https://doi.org/10.4161/auto.25804.
Li E, Li X, Huang J, Xu C, Liang Q, Ren K, et al. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell. 2020;11(9):661–79. https://doi.org/10.1007/s13238-020-00713-x.
da Silva Rosa SC, Martens MD, Field JT, Nguyen L, Kereliuk SM, Hai Y, et al. BNIP3L/Nix-induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation. Autophagy. 2021;17(9):2257–72. https://doi.org/10.1080/15548627.2020.1821548.
Wang J, Gao S, Wang S, Xu Z, Wei L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int J Nanomedicine. 2018;13:3441–50. https://doi.org/10.2147/IJN.S165699.
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138(20):2247–62. https://doi.org/10.1161/CIRCULATIONAHA.117.032821.
Cen X, Chen Y, Xu X, Wu R, He F, Zhao Q, et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nat Commun. 2020;11(1):5731. https://doi.org/10.1038/s41467-020-19547-6.
Boyle KA, Van Wickle J, Hill RB, Marchese A, Kalyanaraman B, Dwinell MB. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J Biol Chem. 2018;293(38):14891–904. https://doi.org/10.1074/jbc.RA117.001469.
Tsang T, Posimo JM, Gudiel AA, Cicchini M, Feldser DM, Brady DC. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell Biol. 2020;22(4):412–24. https://doi.org/10.1038/s41556-020-0481-4.
Yang F, Liao J, Yu W, Qiao N, Guo J, Han Q, et al. Exposure to copper induces mitochondria-mediated apoptosis by inhibiting mitophagy and the PINK1/parkin pathway in chicken (Gallus gallus) livers. J Hazard Mater. 2021;408: 124888. https://doi.org/10.1016/j.jhazmat.2020.124888.
Whang MI, Tavares RM, Benjamin DI, Kattah MG, Advincula R, Nomura DK, et al. The ubiquitin binding protein TAX1BP1 mediates autophagasome induction and the metabolic transition of activated T cells. Immunity. 2017;46(3):405–20. https://doi.org/10.1016/j.immuni.2017.02.018.
Han QA, Yan C, Wang L, Li G, Xu Y, **a X. Urolithin A attenuates ox-LDL-induced endothelial dysfunction partly by modulating microRNA-27 and ERK/PPAR-gamma pathway. Mol Nutr Food Res. 2016;60(9):1933–43. https://doi.org/10.1002/mnfr.201500827.
Raj SD, Fann DY, Wong E, Kennedy BK. Natural products as geroprotectors: an autophagy perspective. Med Res Rev. 2021;41(6):3118–55. https://doi.org/10.1002/med.21815.
Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45: 102049. https://doi.org/10.1016/j.redox.2021.102049.
Ghosh N, Das A, Biswas N, Gnyawali S, Singh K, Gorain M, et al. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD(+) and SIRT1. Sci Rep. 2020;10(1):20184. https://doi.org/10.1038/s41598-020-76564-7.
Acknowledgements
We thank **aoyun Dou for her help in confocal microscopy and appreciate all Dongsheng lab members for their help in experiments.
Funding
This work was supported partly by the National Natural Science Foundation of China (81500343 and 81903358), the Chongqing Talents: Exceptional Young Talents Project (CQYC2020058650), the Natural Science Foundation of Chongqing (cstc2018jcyjAX0355, cstc2020jcyj-msxmX0155 and cstc2021ycjh-bgzxm0105), the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN201900421, KJQN202000423, KJCXZD2020020 and KJQN202100405) and Graduate top-notch program of Chongqing Medical University (BJRC202130). Jun Zhang, Zhen Zou and Chengzhi Chen are supported by Chongqing Bayu Talented Young Scholar program.
Author information
Authors and Affiliations
Contributions
JZ and ZZ conceived and designed this project. YF, ZC, NL, MZ, PW, LZ, XD and SH contributed to major experiments. LM and GX helped in electron microscopy and confocal microscope. XQ, XJ and CC helped to data analysis. JZ and ZZ wrote manuscript with input from all authors. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study has been officially approved by Chongqing Medical University and the protocol was performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Chongqing Medical University. The proof/certificate of approval for animal experiments is available upon request.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, Y., Cheng, Z., Mao, L. et al. PINK1/TAX1BP1-directed mitophagy attenuates vascular endothelial injury induced by copper oxide nanoparticles. J Nanobiotechnol 20, 149 (2022). https://doi.org/10.1186/s12951-022-01338-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01338-4