Abstract
Background
The role of CARM1 in tumors is inconsistent. It acts as an oncogene in most cancers but it inhibits the progression of liver and pancreatic cancers. CARM1 has recently been reported to regulate autophagy, but this function is also context-dependent. However, the effect of CARM1 on gastric cancer (GC) has not been studied. We aimed to explore whether CARM1 was involved in the progression of GC by regulating autophagy.
Methods
The clinical values of CARM1 and autophagy in GC were evaluated by immunohistochemistry and qRT–PCR. Transmission electron microscopy, immunofluorescence and western blotting were employed to identify autophagy. The role of CARM1 in GC was investigated by CCK-8, colony formation and flow cytometry assays in vitro and a xenograft model in vivo. Immunoprecipitation assays were performed to determine the interaction of CARM1 and TFE3.
Results
CARM1 was upregulated in clinical GC tissues and cell lines, and higher CARM1 expression predicted worse prognosis. CARM1 enhanced GC cell proliferation, facilitated G1-S transition and inhibited ER stress-induced apoptosis by regulating autophagy. Importantly, treatment with a CARM1 inhibitor rescued the tumor-promoting effects of CARM1 both in vitro and in vivo. Furthermore, we demonstrated that CARM1 promoted TFE3 nuclear translocation to induce autophagy through the cytoplasmic AMPK-mTOR and nuclear AMPK-CARM1-TFE3 signaling pathways.
Conclusion
CARM1 promoted GC cell proliferation, accelerated G1-S transition and reduced ER stress-induced apoptosis by regulating autophagy. Mechanistically, CARM1 triggered autophagy by facilitating TFE3 nuclear translocation through the AMPK-mTOR and AMPK-CARM1-TFE3 signaling pathways.
Similar content being viewed by others
Introduction
Gastric cancer(GC) is the fifth most commonly diagnosed malignant tumor and the fourth leading cause of cancer death globally [1], causing a severe economic burden. Multidisciplinary treatment has become the mainstay for GC patients, especially with the development of immunotherapy and targeted therapy [2]. However, the number of patients benefiting from the new treatment is limited due to the heterogeneity of the patient population. Therefore, it is critical to investigate the fundamental molecular mechanisms of GC pathogenesis and identify new diagnostic biomarkers and therapeutic targets.
Autophagy is a self-eating process that recycles wastes of cells and maintains homeostasis by clearing longevity proteins or damaged organelles. These cellular contents and organelles are sequestered in double-membrane structures called autophagosomes, which are then fused with lysosomes and degraded [3]. Autophagy is activated when cells encounter environmental stress, such as malnutrition, hypoxia, pathogen infection, and oxidative stress, and cells either adapt or die depending on the intensity of the stimulus and the response of the host [4]. Autophagy is reported to exhibit crucial but conflicting functions in the progression of various tumors, including pancreatic adenocarcinoma, myeloid leukemia, gastric carcinoma, squamous cell carcinoma, sarcoma, and multiple myeloma [5,6,7,4K).
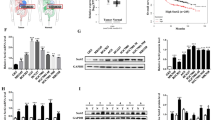
CARM1 promotes the proliferation of GC cells through regulation of autophagy. A CCK-8 assay revealed that downregulation of CARM1 significantly suppressed the growth rate in HGC27 cells. B CARM1-knockdown and control HGC27 cells were treated with rapamycin (50 nM, 24 h). Cell viability was determined by CCK-8 assay. C CCK-8 assay proved that upregulation of CARM1 considerably enhanced cell viability in BGC823 cells. D CARM1-overexpressing and control BGC823 cells were treated with HCQ (25 μM, 24 h). Cell viability was determined by CCK-8 assay. E and F BGC823 cells stably transfected with overexpression or control lentivirus were treated with HCQ (25 μM) 3 days after cells were seeded. Cells were cultured for 10–14 days until visible clones were formed. G and H, HGC27 cells were transfected with CARM1 siRNA every 5 days to maintain the knockdown effect. Cells were cultured in medium containing rapamycin (50 nM) for 10–14 days until visible clones were formed. Representative images of clones are shown from three independent experiments. I Photographs of dissected xenograft tumors showed that the overexpression of CARM1 (top) promoted tumor growth in vivo compared to controls (bottom). J Tumor volumes at the indicated time points were evaluated by the formula: tumor volume (mm3) = [length (mm) × width (mm)2] × π/6. K Tumor weights indicated that the xenograft tumors derived from CARM1-overexpressing cells grew faster than those injected with control gastric cancer cells. Data are presented as the mean ± SD. *Represents *P < 0.05, **P < 0.01 and ***P < 0.001
CARM1 facilitates G1-S cell cycle transition and restrains apoptosis of GC cells by inducing autophagy
To investigate how CARM1 affects cell proliferation by regulating autophagy, we performed a cell cycle assay by flow cytometry. Figure 5A, B shows that downregulation of CARM1 increased while upregulation decreased the proportion of cells in G0/G1 phase, accompanied by a corresponding reduction (CARM1 downregulation) and increase (CARM1 upregulation) in S phase. Treatment with rapamycin partially recovered the decrease in S phase in CARM1-knockdown HGC27 cells, and HCQ reversed the increase in S phase in CARM1-overexpressing cells to some extent. 3-MA also partially reversed the increase in S phase in CARM1-overexpressing BGC823 cells (Additional file 2: Fig. S2D). These results proved that CARM1 accentuated G1-S transition by regulating autophagy.
CARM1 potentiates the G1-S transition of the cell cycle and inhibits the apoptosis of GC cells by inducing autophagy. HGC27 cells transfected with si-CARM1 were treated with rapamycin (50 nM, 24 h), and BGC823 cells transduced with overexpression and control lentiviruses were treated with HCQ (25 μM, 24 h) before analysis by flow cytometry. A and B Distribution of cells in different cell cycle phases determined by flow cytometry is shown for the indicated cells. CARM1 overexpression promoted while CARM1 knockdown attenuated the G1-to-S transition of GC cells. However, the effects could be reversed by the autophagy activator rapamycin and the autophagy inhibitor HCQ. C and D Representative images showing the percentage of cells undergoing apoptosis from three dependent experiments. Treatment with rapamycin partially reversed the increase in apoptosis in CARM1-knockdown HGC27 cells (left panel), and HCQ recovered the decrease in apoptosis in CARM1-overexpressing cells to some extent (right panel). Bars represent the mean ± SD from three independent experiments. *Represents *P < 0.05, **P < 0.01 and ***P < 0.001
Furthermore, apoptosis also plays an important role in the growth of tumor cells. More importantly, as shown in Fig. 3A, B, blockade of autophagy by silencing CARM1 provoked ER stress and subsequently promoted the expression of CHOP and cleaved-caspase3, which are the vital proapoptotic effectors [33]. Therefore, we detected the apoptosis of GC cells under different treatments. As expected, the apoptosis rate was enhanced in CARM1-silenced HGC27 cells, which could be counteracted by rapamycin (Fig. 5C). Opposite effects were confirmed in CARM1-overexpressing cells (Fig. 5D and Additional file 2: Fig. S2E). Therefore, we confirmed that the inhibition of CARM1 lead to the deficient of autophagy flow, resulting in the increase of ER stress-related apoptosis.
CARM1 inhibitor attenuates the tumor-promoting effect of CARM1
Given that CARM1 could promote GC tumor growth both in vitro and in vivo, we sought to investigate whether the CARM1 inhibitor (CARM1i) EZM2302 could exert a therapeutic efficacy. As revealed in Fig. 6A, B, CARM1i suppressed the increase in cell viability and clonogenicity induced by CARM1 overexpression. Concordantly, CARM1i also triggered G1 phase cell cycle arrest and significantly reversed the decreased apoptosis rate in CARM1-elevated cells (Fig. 6C, D). Next, we tested the therapeutic effect of CARM1i in mouse xenograft models in vivo, and we found that CARM1i had a beneficial therapeutic effect, as demonstrated by the reduced growth rate and eventual reduction in tumor volume, as well as the decreased tumor weight compared to the untreated group injected with CARM1-overexpressing cells (Fig. 6E–G). Interestingly, CARM1i exhibited a synergistic effect when administered in combination with HCQ, suggesting a potential therapeutic strategy for GC (Fig. 6E–G).
CARM1 inhibitor rescued the tumor-promoting role of CARM1 both in vitro and in vivo. A Control and CARM1-overexpressing BGC823 cells were treated with CARM1i (8 μM, 24 h), and cell viability was determined by CCK-8 assay. B Control and CARM1-overexpressing BGC823 cells were cultivated in medium containing 8 μM CARM1i for 10–14 days. CARM1i reduced the increase in colony numbers caused by CARM1 overexpression. C and D Control and CARM1-overexpressing BGC823 cells were treated with CARM1i (8 μM, 24 h). Cell cycle phase distribution and apoptosis in the indicated cells were assessed by flow cytometry. E, F and G BGC823 xenograft models were generated to investigate the therapeutic effect of CARM1i and HCQ on GC in vivo. Nude mice injected with CARM1-overexpressing cells were randomly divided into four groups on day 6 when tumor volumes reached 50 mm3. CARM1i injection was performed twice daily at 100 mg/kg i.p. HCQ was administered once daily at 50 mg/kg i.p. The combination group was given two treatments, and the control group was intraperitoneally injected with PBS only. E Photograph of subcutaneous tumors dissected from animals in the indicated groups. F The tumor volumes were measured every four days and calculated by the formula: tumor volume (mm3) = [length (mm) × width (mm)2] × π/6. G The tumor weights were evaluated. Both CARM1i and HCQ slowed the tumor growth of GC. Furthermore, CARM1i exhibited a synergistic effect in combination with HCQ. Data are demonstrated as the mean ± SD. *Represents *P < 0.05, **P < 0.01 and ***P < 0.001
CARM1 activates autophagy by promoting TFE3 nuclear translocation
Previous studies have shown that the MiTF/TFE family, including TFE3, TFEB, MITF and TFEC, plays important roles in various physiological processes, especially in lysosomal homeostasis and autophagy regulation [34, 35]. Because TFEC is mainly expressed in bone marrow-derived cells [36], we examined the expression of MITF, TFEB and TFE3 to explore whether the MiTF/TFE family was involved in the regulation of autophagy by CARM1. As shown in Fig. 7A, MITF exhibited no difference, while TFEB and TFE3 were attenuated in CARM1-silenced cells and increased in CARM1-overexpressing cells. CARM1 has been reported to interact with TFEB as its coactivator to promote the transcription of autophagy-related genes [26], and TFE3 and TFEB could share common regulatory networks [37]. Therefore, this study mainly focused on whether CARM1 could promote autophagy by regulating TFE3. Western blotting of nuclear proteins and immunofluorescence staining demonstrated that CARM1 could promote the nuclear translocation of TFE3 (Fig. 7B, C). To illustrate the effect of TFE3 nuclear translocation on autophagy, we knocked down TFE3 expression using siRNA. The TFE3 expression was successfully silenced, and we found that TFE3 knockdown induced a deficiency in autophagy and a subsequent increase in ER stress-mediated apoptotic protein expression (Fig. 7D). Functional experiments further verified that TFE3 knockdown could reverse the increase in cell growth caused by CARM1-induced autophagy (Fig. 7E–G).
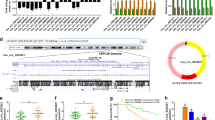
CARM1 activates autophagy by promoting TFE3 nuclear translocation. A The mRNA expression of the MiTF/TFE family (TFE3, TFEB, MITF) was detected by qRT–PCR in CARM1-knockdown HGC27 cells and CARM1-overexpressing BGC823 cells. B Western blotting was used to assess TFE3 protein levels in the nucleus of CARM1-treated cells, and histone H3 was used as a loading control for nuclear protein. C The percentage of TFE3 translocation into the nucleus was evaluated by immunofluorescence (scale bar: 50 μm). D Decreased TFE3 expression in CARM1-overexpressing BGC823 cells blocked autophagy flux and subsequently induced ER stress. Autophagy- and ER stress-related protein expression was evaluated in the cytoplasm, and TFE3 expression was examined in the nucleus by western blotting. β-Actin and H3 served as loading controls for cytoplasmic proteins and nuclear proteins, respectively. E and F Colony formation and CCK-8 assays revealed that TFE3 downregulation reversed the pro-proliferative effect of CARM1 on BGC823 cells. G TFE3 knockdown rescued the increased G1-S transition and decreased apoptosis resulting from CARM1 overexpression in BGC823 cells. Bars represent the mean ± SD from three independent experiments. *Represents *P < 0.05, **P < 0.01 and ***P < 0.001
The TFE3 activity is activated through AMPK-mTOR and AMPK-CARM1-TFE3 signaling pathways
We next tried to clarify the mechanisms by which increased CARM1 promoted the nuclear translocation of TFE3. Previous studies have shown that AMPK-mTOR is an important pathway involved in the regulation of the MiTF/TFE family [34]. Our results demonstrated that in the cytoplasm, p-AMPK/AMPK expression was enhanced while p-mTOR/mTOR was reduced, and that TFE3 nuclear expression was increased in CARM1-overexpressing cells (Fig. 8A). These observations suggested that TFE3 activity was partially regulated by the cytoplasmic AMPK-mTOR signaling pathway. It has been reported that CARM1 can bind to the promoter region of TFEB to enhance transcription [26]. Since TFE3 always shares a regulatory mechanism with TFEB, we conducted an immunoprecipitation assay to explore whether CARM1 could also interact with TFE3. As exhibited in Fig. 8B, the overexpression of CARM1 increased the TFE3 binding level, regardless of different CARM1 expression levels (Fig. 8B). To further investigate the effect of AMPK on TFE3, we applied Compound C, an effective reversible inhibitor of AMPK [38]. As shown in Fig. 8A, B, Compound C suppressed the cytoplasmic AMPK-mTOR pathway and reduced both TFE3 nuclear translocation and binding activity to CARM1.
The TFE3 activity is activated via the cytoplasmic AMPK-mTOR and nuclear AMPK-CARM1-TFE3 signaling pathways. A Western blotting analysis of the AMPK-mTOR signaling pathway in the cytoplasm and TFE3 expression in the nucleus in the CARM1-EV, CARM1-OE, CARM1-OE + DMSO, and CARM1-OE + Compound C (10 μM, 6 h) cells. B CARM1-overexpressing BGC823cells were treated with Compound C (10 μM, 6 h) or the solvent control DMSO. An immunoprecipitation assay using a CARM1 antibody was performed, and the amount of TFE3 protein bound to CARM1 under different experimental conditions was evaluated by western blotting. Values represent the means ± SD. **P < 0.01 and ***P < 0.001. C Schematic model for the mechanisms of function of CARM1 in GC. CARM1 potentiates autophagy by facilitating TFE3 nuclear translocation and enhancing its activity by activating cytosolic AMPK-mTOR and nuclear AMPK-CARM1-TFE3 signaling pathways. Then, CARM1 promotes GC cell proliferation and reduces ER stress-induced apoptosis by regulating autophagy
Discussion
GC remains one of the deadliest malignancies, and there is a lack of effective early diagnosis and prognostic molecular markers. CARM1 is elevated in various tumors and exhibits a tumor-promoting role mainly as a transcriptional coactivator by methylating histones and non-histones [25, 39]. Paradoxically, CARM1 shows a tumor-inhibiting effect on liver and pancreatic cancers [19, 20]. However, the role of CARM1 and the associated mechanism of pathogenesis in GC have not been studied before. Here, we demonstrated that CARM1 expression was increased by analyzing data in databases and clinical samples of GC and proved for the first time that CARM1 was an independent risk factor for predicting poor survival (Fig. 1A–D). These findings indicate that CARM1 may potentially become a marker for diagnosis and therapeutic decision making for GC.
Recent studies have shown the vital role of CARM1 in autophagy [11]. Autophagy maintains cellular homeostasis through the degradation of long-lived proteins and damaged organelles, and is considered to be a self-defense mechanism against certain stressful conditions [40]. The role of autophagy in tumors is paradoxical depending on the specific environment [41, 42]. On the one hand, autophagy markers are often located in cancer-related DNA regions with frequent mutations or deletions which may be protective mechanisms to inhibit tumor initiation [43], and it has been proven that 5-FU can inhibit GC cell growth and survival by inducing autophagy-related death [44]. On the other hand, autophagy can help tumor cells resist nutritional deficiencies and other cancer-associated conditions to promote tumor progression and chemotherapy resistance [45]. In this study, we demonstrated that CARM1 promoted GC cell proliferation through autophagy-regulated G1-S transition (Figs. 4A–H, 5A, B).
Furthermore, our results showed that defective autophagy process caused by CARM1 inhibition contributed to ER stress-related apoptosis, which also affected tumor growth. There are three main UPR signaling pathways initiated by IRE1α, PERK, and ATF6, respectively, to reduce protein load and enhance protein-folding capacity [46], and we found that the ER stress initiated by CARM1-mediated autophagy was regulated mainly by the PERK pathway (Fig. 3B, C). PERK undergoes dimerization and autophosphorylation, which activates its kinase domain and subsequent phosphorylation of eIF2α. p-eIF2α relieves the unfolded protein burden of ER [47] by inhibiting most protein translation but selectively enhancing ATF4 translation, leading to an increase in downstream protein CHOP expression [48]. CHOP is responsible for the apoptosis of cells with dysfunctional ER [49]. In our study, increased ER stress mediated by impaired autophagy promoted CHOP expression and eventually potentiated apoptosis (Fig. 5C).
Our results demonstrated that CARM1 exerted a significant tumor-promoting role in GC, therefore, we sought to explore the therapeutic effect of targeting CARM1 by utilizing the small molecule compound EZM2302, which is a specific inhibitor of CARM1 and has shown a significant inhibitory effect on multiple myeloma both in vitro and in vivo [21]. In mouse models of AML and diffuse large B-cell lymphoma, CARM1i also exhibited an effective inhibitory effect [50, 51]. Hence, we wished to determine whether CARM1i could also inhibit the progression of GC, a solid tumor. As shown in Fig. 6A–D, CARM1i retarded GC cell viability, induced G1 cell cycle arrest and triggered apoptosis in vitro, and the growth of xenograft tumors in vivo were also suppressed (Fig. 6E–G). Furthermore, the autophagy inhibitor HCQ, which has been proven to possess appreciable antitumor activity in clinical trials [52, 53], demonstrated synergistic antitumor effects with CARM1i, suggesting the combination of CARM1i and HCQ as a potential therapeutic strategy.
Finally, we aimed to clarify how CARM1 regulated autophagy. The MiT/TFE family was reported to exert an essential function in autophagy regulation [54]. We found that TFEB and TFE3 were both increased in CARM1-overexpressing cells (Fig. 7A). As TFEB has been clearly demonstrated to interact with CARM1 to promote autophagy [26], we focused our study on whether CARM1 could induce autophagy by regulating TFE3. TFE3 helps maintain lysosomal homeostasis and promotes autolysosome formation by binding to the CLEAR elements of lysosome- and autophagy-relevant genes [55]. The results showed that CARM1 promoted TFE3 nuclear translocation to activate autophagy, and silencing TFE3 reversed the increased cell proliferation and decreased apoptosis induced by CARM1-mediated autophagy. Then, we further explored the mechanisms by which increased CARM1 promoted the nuclear translocation of TFE3. AMPK is a responder to starvation and low energy states to maintain intracellular energy homeostasis. Previous studies have shown that AMPK is often activated during the development of GC, demonstrating a pro-tumor effect [56, 57]. AMPK is considered to be a key signaling pathway regulating the activity of the MiT/TFE transcription factor family [34]. It has been reported that TFE3 nuclear translocation is closely related to the activation of the AMPK-mTOR signaling pathway in the cytoplasm. As reported, MTOR complex 1 (MTORC1) is the most important regulator of TFE3 which directly phosphorylates TFE3 serine residue 321 and causes TFE3 cytoplasmic retention [58]. MTORC1 activity is negatively regulated by AMPK through direct and indirect phosphorylation under energy deficiency [59, 60]. Moreover, activated AMPK could directly phosphorylate TFE3 serine residues and regulate TFE3 transcriptional activity. Therefore, we investigated the cytoplasmic AMPK-mTOR pathway and found that the activated AMPK-mTOR pathway was responsible for the nuclear translocation of TFE3 (Fig. 8A). Furthermore, AMPK also regulates the MiT/TFE family through nuclear AMPK-CARM1 signaling pathway. When activated in the nucleus, AMPK phosphorylates FOXO3a and transcriptionally inhibits SKP2, thereby reducing the ubiquitination of CARM1 and increasing the protein level of CARM1 in the nucleus [26]. CARM1 was reported to coactivate TFEB in the nucleus, and TFEB and TFE3 always shared regulatory networks. We then explored the interaction of CARM1 and TFE3 in the nucleus and proved that CARM1 could also regulate autophagy through the nuclear AMPK-CARM1-TFE3 signaling pathway (Fig. 8B). Furthermore, it has been reported that CARM1 can regulate the activation of AMPK in skeletal muscle during denervation-induced plasticity. CARM1 interacts with AMPK and causes the methylation of AMPK, resulting in AMPK activation [61]. This result is consistent with the increased AMPK activation in CARM1-overexpressing cells in our study. These results suggest that there may be a regulatory loop between CARM1 and AMPK which forms positive feedback to promote tumor development. And the detailed mechanism and structure–function relationship are needed to be further explored.
In conclusion, the present study first demonstrated that CARM1 was upregulated in clinical GC tissues and cell lines, and that higher CARM1 expression was related to an unfavorable prognosis. More importantly, our study proved for the first time that CARM1i treatment significantly inhibited GC tumor growth both in vivo and in vitro and synergized with autophagy inhibitors, suggesting a promising therapeutic strategy. CARM1 promoted GC cell proliferation, accelerated G1-S transition and reduced ER stress-induced apoptosis by regulating autophagy. Mechanistically, CARM1 potentiated autophagy by facilitating TFE3 nuclear translocation and enhancing its activity through the activation of cytosolic AMPK-mTOR and nuclear AMPK-CARM1-TFE3 signaling pathways.
Availability of data and materials
The data and materials supporting the findings of the research are available from the corresponding author.
Abbreviations
- GC:
-
Gastric cancer
- CARM1:
-
Coactivator-associated arginine methyltransferase-1
- IHC:
-
Immunohistochemistry
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ER stress:
-
Endoplasmic reticulum stress
- MITF:
-
Melanocyte inducing transcription factor
- TFE3:
-
Transcription factor binding to IGHM enhancer 3
- TFEB:
-
Transcription factor EB
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–79.
Yang ZF, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–31.
He CC, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
Altman JK, Szilard A, Goussetis DJ, Sassano A, Colamonici M, Gounaris E, et al. Autophagy is a survival mechanism of acute myelogenous leukemia precursors during dual mTORC2/mTORC1 targeting. Clin Cancer Res. 2014;20(9):2400–9.
Kun Z, Hanqing G, Hailing T, Yuan Y, Jun Z, Lingxia Z, et al. Gastrin enhances autophagy and promotes gastric carcinoma proliferation via inducing AMPKα. Oncol Res. 2017;25(8):1399–407.
Masui A, Hamada M, Kameyama H, Wakabayashi K, Takasu A, Imai T, et al. Autophagy as a survival mechanism for squamous cell carcinoma cells in endonuclease G-mediated apoptosis. PLoS ONE. 2016;11(9):e0162786.
Wang Y, **ong H, Liu D, Hill C, Ertay A, Li J, et al. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy. 2019;15(5):886–99.
Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–18.
Baek SH, Kim KI. Epigenetic control of autophagy: nuclear events gain more attention. Mol Cell. 2017;65(5):781–5.
Suresh S, Huard S, Dubois T. CARM1/PRMT4: making its mark beyond its function as a transcriptional coactivator. Trends Cell Biol. 2021;31(5):402–17.
Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33(1):1–13.
Kim JH, Yoo BC, Yang WS, Kim E, Hong S, Cho JY. The role of protein arginine methyltransferases in inflammatory responses. Mediators Inflamm. 2016;2016:4028353.
Al-Dhaheri M, Wu J, Skliris GP, Li J, Higashimato K, Wang Y, et al. CARM1 is an important determinant of ERalpha-dependent breast cancer cell differentiation and proliferation in breast cancer cells. Cancer Res. 2011;71(6):2118–28.
Elakoum R, Gauchotte G, Oussalah A, Wissler MP, Clement-Duchene C, Vignaud JM, et al. CARM1 and PRMT1 are dysregulated in lung cancer without hierarchical features. Biochimie. 2014;97:210–8.
Karakashev S, Zhu H, Wu S, Yokoyama Y, Bitler BG, Park PH, et al. CARM1-expressing ovarian cancer depends on the histone methyltransferase EZH2 activity. Nat Commun. 2018;9(1):631.
Majumder S, Liu Y, Ford OH 3rd, Mohler JL, Whang YE. Involvement of arginine methyltransferase CARM1 in androgen receptor function and prostate cancer cell viability. Prostate. 2006;66(12):1292–301.
Ou CY, LaBonte MJ, Manegold PC, So AY, Ianculescu I, Gerke DS, et al. A coactivator role of CARM1 in the dysregulation of beta-catenin activity in colorectal cancer cell growth and gene expression. Mol Cancer Res. 2011;9(5):660–70.
Wang YP, Zhou W, Wang J, Huang X, Zuo Y, Wang TS, et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol Cell. 2016;64(4):673–87.
Zhong XY, Yuan XM, Xu YY, Yin M, Yan WW, Zou SW, et al. CARM1 methylates GAPDH to regulate glucose metabolism and is suppressed in liver cancer. Cell Rep. 2018;24(12):3207–23.
Drew AE, Moradei O, Jacques SL, Rioux N, Boriack-Sjodin AP, Allain C, et al. Identification of a CARM1 inhibitor with potent in vitro and in vivo activity in preclinical models of multiple myeloma. Sci Rep. 2017;7(1):17993.
Greenblatt SM, Man N, Hamard PJ, Asai T, Karl D, Martinez C, et al. CARM1 is essential for myeloid leukemogenesis but dispensable for normal hematopoiesis. Cancer Cell. 2019;35(1):156.
Li S, Cheng D, Zhu B, Yang Q. The overexpression of CARM1 promotes human osteosarcoma cell proliferation through the pGSK3beta/beta-catenin/cyclinD1 signaling pathway. Int J Biol Sci. 2017;13(8):976–84.
Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20(10):642–57.
Jarrold J, Davies CC. PRMTs and arginine methylation: cancer’s best-kept secret? Trends Mol Med. 2019;25(11):993–1009.
Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, et al. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534(7608):553–7.
Liu Y, Wang T, Ji YJ, Johnson K, Liu H, Johnson K, et al. A C9orf72-CARM1 axis regulates lipid metabolism under glucose starvation-induced nutrient stress. Genes Dev. 2018;32(21–22):1380–97.
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–31.
Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer. 2016;2(5):252–62.
Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN. Localization of the gene encoding human BiP/GRP78, the endoplasmic reticulum cognate of the HSP70 family, to chromosome 9q34. Genomics. 1994;20(2):281–4.
Wang XJ, Yu J, Wong SH, Cheng AS, Chan FK, Ng SS, et al. A novel crosstalk between two major protein degradation systems: regulation of proteasomal activity by autophagy. Autophagy. 2013;9(10):1500–8.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–45.
McComb S, Chan PK, Guinot A, Hartmannsdottir H, Jenni S, Dobay MP, et al. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci Adv. 2019;5(7):eaau9433.
Slade L, Pulinilkunnil T. The MiTF/TFE family of transcription factors: master regulators of organelle signaling, metabolism, and stress adaptation. Mol Cancer Res. 2017;15(12):1637–43.
Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524(7565):361–5.
Rehli M, Lichanska A, Cassady AI, Ostrowski MC, Hume DA. TFEC is a macrophage-restricted member of the microphthalmia-TFE subfamily of basic helix-loop-helix leucine zipper transcription factors. J Immunol. 1999;162(3):1559–65.
Martina JA, Puertollano R. TFEB and TFE3: the art of multi-tasking under stress conditions. Transcription. 2017;8(1):48–54.
Abdulrahman RM, Boon MR, Sips HC, Guigas B, Rensen PC, Smit JW, et al. Impact of Metformin and compound C on NIS expression and iodine uptake in vitro and in vivo: a role for CRE in AMPK modulation of thyroid function. Thyroid. 2014;24(1):78–87.
Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18(3):263–72.
Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB 3rd. Autophagy: regulation and role in development. Autophagy. 2013;9(7):951–72.
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen GH, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–75.
White E. The role for autophagy in cancer. J Clin Investig. 2015;125(1):42–6.
Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59(1):59–65.
Yang C, Pan Y. Fluorouracil induces autophagy-related gastric carcinoma cell death through Beclin-1 upregulation by miR-30 suppression. Tumour Biol. 2015;37:15489.
Cao Y, Luo Y, Zou J, Ouyang J, Cai Z, Zeng X, et al. Autophagy and its role in gastric cancer. Clin Chim Acta. 2019;489:10–20.
Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):169–81.
Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25(5):563–73.
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108.
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95.
Greenblatt SM, Man N, Hamard PJ, Asai T, Karl D, Martinez C, et al. CARM1 is essential for myeloid leukemogenesis but dispensable for normal hematopoiesis. Cancer Cell. 2018;33(6):1111-27.e5.
Veazey KJ, Cheng D, Lin K, Villarreal OD, Gao G, Perez-Oquendo M, et al. CARM1 inhibition reduces histone acetyltransferase activity causing synthetic lethality in CREBBP/EP300-mutated lymphomas. Leukemia. 2020;34(12):3269–85.
Rojas-Puentes LL, Gonzalez-Pinedo M, Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nuñez-Gomez R, et al. Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases. Radiat Oncol (London, England). 2013;8:209.
Dong X, Wang Y, Zhou Y, Wen J, Wang S, Shen L. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discovery. 2016;2:16087.
Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M, et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet. 2011;20(19):3852–66.
Lim JA, Meena NK, Raben N. Pros and cons of different ways to address dysfunctional autophagy in Pompe disease. Ann Transl Med. 2019;7(13):279.
Thirupathi A, Chang YZ. Role of AMPK and its molecular intermediates in subjugating cancer survival mechanism. Life Sci. 2019;227:30–8.
Kim YH, Liang H, Liu X, Lee JS, Cho JY, Cheong JH, et al. AMPKα modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72(10):2512–21.
Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–62.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–68.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26.
Stouth DW, vanLieshout TL, Ng SY, Webb EK, Manta A, Moll Z, et al. CARM1 regulates AMPK signaling in skeletal muscle. iScience. 2020;23(11):101755.
Acknowledgements
We gratefully acknowledge all supports from participants in this research.
Funding
The study was funded by Shanxi Provincial Science & Technology Plan Project (2016KTZDSF02‐02), National Natural Science Foundation of China (No.82001079), Institutional Foundation of the First Affiliated Hospital of **’an Jiaotong University (2020QN-26) and ****g Hospital Boost Plan (XJZT19MJ23).
Author information
Authors and Affiliations
Contributions
SZY, JZ, DC and JYC carried out the experimental, collected and analyzed the data, assembled the figures and wrote the initial article. YZ, YYH, YRJ and SHW helped perform the experiments and generated data. TW, LM, TTL contributed to clinical sample collection. YW, WQ, LD designed experiments, provided key support and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experiments were approved by the Animal Ethics Committee of **’an Jiaotong University. All animal experiments follow the guidelines stipulated by the Medical School of **’an Jiaotong University (G-222).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
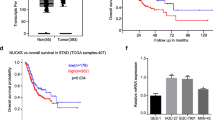
A Gene expression profile across all tumor samples and paired normal tissues from the GEPIA database. CARM1 expression was increased in gastric cancer tissues compared to normal tissues (indicated by the red box). B, Data from the KM Plotter database suggested that overall survival was shorter in GC patients with higher CARM1 expression. C, The IRE1α and ATF6α protein expression levels were evaluated by western blotting. Bars represent the mean ± SD from three independent experiments.
Additional file 2: Figure S2.
Autophagy inhibitor 3-MA rescued the tumor-promoting role of CARM1. A Protein levels of p62 and LC3BII/I were determined by western blotting using β-Actin as an internal control. B Control and CARM1-overexpressing BGC823 cells were treated with 3-MA (5 mM, 24 h), and cell viability was examined by CCK-8 assay. C BGC823 cells stably transfected with overexpression or control lentivirus were treated with3-MA (5 mM) 4 days after cells were seeded. Cells were cultured for 10–14 days until visible clones were formed. The colony formation assay revealed that 3-MA reversed the pro-proliferative effect caused by CARM1 overexpression. D and E Control and CARM1-overexpressing BGC823 cells were treated with 3-MA (5 mM, 24 h). D Distribution in different cell cycle phases was analyzed for the indicated cells by flow cytometry. E The percentage of cells undergoing apoptosis was determined by flow cytometry. Data are expressed as the mean ± SD. *Represents *P < 0.05, **P < 0.01 and ***P < 0.001.
Additional file 3: Table S1.
Association of CARM1 level with clinicopathological parameters of patients with gastric cancer.
Additional file 4: Table S2.
Sequences of primers and targets for siRNA.
Additional file 5: Table S3.
Antibodies and regents used in experiments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Zhang, J., Chen, D. et al. CARM1 promotes gastric cancer progression by regulating TFE3 mediated autophagy enhancement through the cytoplasmic AMPK-mTOR and nuclear AMPK-CARM1-TFE3 signaling pathways. Cancer Cell Int 22, 102 (2022). https://doi.org/10.1186/s12935-022-02522-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02522-0