Abstract
Background
Statin treatment increases the risk of new-onset diabetes mellitus (NODM); however, data directly comparing the risk of NODM among individual statins is limited. We compared the risk of NODM between patients using pitavastatin and atorvastatin or rosuvastatin using reliable, large-scale data.
Methods
Data of electronic health records from ten hospitals converted to the Observational Medical Outcomes Partnership Common Data Model (n = 14,605,368 patients) were used to identify new users of pitavastatin, atorvastatin, or rosuvastatin (atorvastatin + rosuvastatin) for ≥ 180 days without a previous history of diabetes or HbA1c level ≥ 5.7%. We conducted a cohort study using Cox regression analysis to examine the hazard ratio (HR) of NODM after propensity score matching (PSM) and then performed an aggregate meta-analysis of the HR.
Results
After 1:2 PSM, 10,238 new pitavastatin users (15,998 person-years of follow-up) and 18,605 atorvastatin + rosuvastatin users (33,477 person-years of follow-up) were pooled from 10 databases. The meta-analysis of the HRs demonstrated that pitavastatin resulted in a significantly reduced risk of NODM than atorvastatin + rosuvastatin (HR 0.72; 95% CI 0.59–0.87). In sub-analysis, pitavastatin was associated with a lower risk of NODM than atorvastatin or rosuvastatin after 1:1 PSM (HR 0.69; CI 0.54–0.88 and HR 0.74; CI 0.55–0.99, respectively). A consistently low risk of NODM in pitavastatin users was observed when compared with low-to-moderate-intensity atorvastatin + rosuvastatin users (HR 0.78; CI 0.62–0.98).
Conclusions
In this retrospective, multicenter active-comparator, new-user, cohort study, pitavastatin reduced the risk of NODM compared with atorvastatin or rosuvastatin.
Similar content being viewed by others
Background
Statins reduce blood cholesterol levels and are widely used for the primary or secondary prevention of cardiovascular diseases [1, 2]. Although statin treatment is generally considered safe, several recent studies have suggested that it confers an increased risk of new-onset diabetes mellitus (NODM) [3, 4]. Overall, statins have been found to increase the risk of NODM by 10–12%, and the risk is slightly greater with high-intensity statin therapy than with low- or moderate-intensity therapy [4]. However, due to the limited data directly comparing statins, the risk of NODM among individual statins remains controversial. A previous meta-analysis showed no significant difference in diabetic risk among various statins, while a population-based, retrospective, cohort study showed that atorvastatin and simvastatin were associated with an increased risk of NODM compared to pravastatin [3, 5, 6]. Moreover, Yoon et al. reported that the atorvastatin-exposed cohort had the highest risk of NODM compared with the matched, non-exposed cohort among various statins [7]. In contrast, a meta-analysis of 17 studies reported that rosuvastatin was associated with the highest risk of NODM among various statins [8].
Pitavastatin is a highly potent statin along with atorvastatin and rosuvastatin. LDL-C decreased by an average of 47% after treatment with pitavastatin 4 mg, which was comparable to atorvastatin 40 mg or rosuvastatin 10 mg [9]. Previous randomized clinical trial demonstrated that pitavastatin 4 mg compared with pitavastatin 1 mg therapy significantly reduced LDL-C and clinical outcomes irrespective of renal function state [10]. When used in combination, pitavastatin and pemafibrate improved lipid profile and endothelial function in hypertension and insulin resistance model rats [11]. Ihm et al. also reported combination therapy with pitavastatin and fenofibrate more effectively reduced non-HDL-C compared with pitavastatin monotherapy in patients with a high risk for cardiovascular disease [12]. Several previous studies have reported that pitavastatin had less influence on the development of diabetes mellitus or glucose metabolism than other statins, such as pravastatin, atorvastatin, or rosuvastatin [13,14,15,16]. Additionally, pitavastatin tended to have a slightly lower hazard ratio (HR) for NODM than other statins in a real-world cohort study of Asian patients [7]. In contrast, a single-center, retrospective study including 3680 patients reported that pitavastatin was more strongly associated with NODM than other statins [17]. These inconclusive results might be due to limited data for direct comparisons of individual statins and various biases, including selection, immortal, protopathic, and/or confounding bias originating from research design error, relatively small sample size, or analysis method.
In present new-user model cohort study, we assessed the impact of pitavastatin on NODM compared with atorvastatin or rosuvastatin, the most commonly prescribed statins in the world, in patients without diabetes or impaired glucose tolerance using the Observational Medical Outcomes Partnership (OMOP)- Common Data Model (CDM) of large-scale data validated in our previous studies [18,19,20].
Methods
Data sources
This multicenter, controlled cohort study included real-world clinical data of 14,605,368 patients from 10 secondary or tertiary hospitals in Korea converted to the OMOP-CDM version 5.3. The breakdown was as follows: (1) Kangdong Sacred Heart Hospital CDM (KDH; 1,689,604 patients); (2) Kyung Hee University Hospital at Gangdong CDM (KHNMC; 822,183 patients); (3) Wonkwang University Hospital CDM (WKUH; 1,001,794 patients); (4) Daegu Catholic University Medical Center CDM (DCMC; 1,688,980 patients); (5) Ajou University Medical Center CDM (AUMC; 3,109,677 patients); (6) Pusan National University Hospital (PNUH; 1,753,001 patients); (7) Ewha Womans University Mokdong Hospital CDM (EUMC; 1,745,549 patients); (8) National Health Insurance Service Ilsan Hospital CDM (NHIMC; 1,367,483 patients); (9) Myongji Hospital (MJH; 882,646 patients); and (10) Kangwon National University Hospital CDM (KWMC; 544,451 patients). All databases comprise de-identified, patient-level, electronic health record data converted into the standard vocabulary of the CDM to generate network-wide results through distributed research networks using the same analysis program among collaborating organizations [21, 22].
Study design
We conducted a multicenter, retrospective, observational, comparative, new-user cohort study. Patients aged ≥ 18 years who were first exposed to pitavastatin, atorvastatin, or rosuvastatin were included in this study. For consistency in the definition of “new-users” to minimize immortal-time bias, we only included patients who had a continuous observational period of > 365 days prior to the first prescription day of the study drugs and excluded patients with known prior exposure to any other statin (simvastatin, pravastatin, lovastatin, and fluvastatin), including crossover among the study drugs at any time before and within 180 days after the first prescription of the study drug. In this study, 2–4 mg of pitavastatin, 10–80 mg of atorvastatin, and 5–20 mg of rosuvastatin were used. We defined 10–20 mg of atorvastatin and 5–10 mg of rosuvastatin as moderate-intensity statin and 40–80 mg of atorvastatin and 20 mg of rosuvastatin as high-intensity statin according to the recent guidelines [23].
The index date was defined as the first day of study drug prescription. The target cohort was defined as patients who were first prescribed pitavastatin at any dose for > 180 consecutive days. The comparator cohort was defined as patients who were first prescribed atorvastatin or rosuvastatin (atorvastatin + rosuvastatin) at any dose for > 180 consecutive days. Continuous drug exposure was established by allowing gaps < 90 days between prescriptions. The cohort end date was defined as the date of the end of continuous study drug exposure, drug exposure of another statin during follow-up, or ascertainment of NODM. The time at risk of the study was defined from 180 days after the index date to 180 days after the cohort end date. Patients who met at least one of the following criteria at any days before and within 180 days after the index date were also excluded from both cohorts to confirm the absence of diabetes mellitus and impaired glucose tolerance: (1) a diagnosis of diabetes disorder including impaired glucose tolerance; (2) exposure to any oral hypoglycemic agent, glucagon-like pepetide-1 receptor agonists, or insulin; and (3) serum hemoglobin A1c (HbA1c) level ≥ 5.7%.
The primary outcome was the incidence of NODM 180 days after the index date. NODM was defined as the occurrence of at least one of the following criteria between 180 days after the index date and 180 days after the cohort end date: (1) diagnosis of diabetes mellitus as identified by the 10th version of the International Classification of Diseases (ICD); (2) prescription of any hypoglycemic agent, glucagon-like pepetide-1 receptor agonist, or insulin; and (3) serum HbA1c level ≥ 6.5%. The secondary outcomes included (1) the incidence of NODM in each group (any dose of pitavastatin, atorvastatin, and rosuvastatin), (2) the incidence of NODM with any dose of pitavastatin and high-intensity atorvastatin or rosuvastatin, and (3) the incidence of NODM with any dose of pitavastatin and moderate-intensity atorvastatin or rosuvastatin. In the analysis according to the statin intensities, we also excluded patients having dose changes between high and moderate-intensity statin during follow up.
Statistical analysis
We performed our cohort study using the open-source OHDSI CohortMethod R package with large-scale analytics from the Cyclops R package [24, 25]. We used ATLAS version 2.7.5, and the analysis was performed using FEEDER-NET, a Korean health data platform based on the OMOP-CDM. We used large-scale propensity score matching to balance the target and comparator cohorts to reduce potential confounding due to an imbalance in baseline covariates. Covariates used in the propensity score model included age, sex, prior conditions, drugs observed during the 365 days and 30 days prior to study drug exposure, and Romano’s Adaptation of the Charlson comorbidity index [26]. Propensity scores were estimated using large-scale logistic regression models, and greedy search matching was used to match patients with a caliper of 0.2 times for the standard deviation of the propensity score distribution. We performed 1:2 propensity score matching (PSM) to compare pitavastatin with atorvastatin + rosuvastatin. Additionally, 1:1 PSM was used for comparing pitavastatin vs. atorvastatin, pitavastatin vs. rosuvastatin, atorvastatin vs. rosuvastatin, pitavastatin vs. moderate-intensity atorvastatin + rosuvastatin, pitavastatin vs. moderate-intensity atorvastatin, pitavastatin vs. moderate-intensity rosuvastatin, and pitavastatin vs. high-intensity atorvastatin + rosuvastatin. We conducted Cox regression analysis to examine the HR for NODM between the cohorts. Kaplan–Meier analysis was used to estimate the cumulative incidence of NODM. Incidence rates were determined per 1000 person-years by dividing the number of cases of NODM by the total number of person-years at risk. Two-sided P-values < 0.05 were considered statistically significant.
After conducting identical analytic process 10 databases with the single execute-to-end dedicated R package, we aggregated results of 10 databases by meta-analysis. The assessment for statistical tests of heterogeneity was calculated using the χ2 and I2 statistics. When there was no significant result for heterogeneity (P > 0.10, I2 < 50%), a fixed-effects model was used; otherwise, a random-effects model was used. However, we reported results from both, fixed- and random-effects models as a sensitivity analysis. All analyses were performed using R statistical software (version 3.6.1; R Foundation for Statistical Computing).
Results
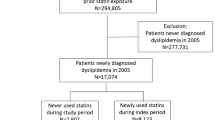
A total of 87,734 patients across the 10 databases were included in the present study (11,396 patients were new users of pitavastatin [17,944 person-years of follow-up] and 76,338 patients were new users of atorvastatin + rosuvastatin [137,966 person-years of follow-up]) (Fig. 1). We performed 1:2 PSM to compare the two groups. From 8714 (PNUH) to 13,546 (AUMC) baseline covariates were matched in 10 databases and most of the standardized mean differences were lesser than 0.1 after PSM (Fig. 2). In total, 10,238 patients treated with pitavastatin (15,998 person-years of follow-up) and 18,605 patients treated with atorvastatin + rosuvastatin (33,477 person-years of follow-up) were pooled after 1:2 PSM.
Table 1 shows the baseline characteristics of patients in the KDH cohort before and after PSM. The atorvastatin + rosuvastatin group had a higher Charlson comorbidity index score and incidence of ischemic heart disease and cerebrovascular disease before PSM, but the baseline covariables were well-balanced after PSM. Across all databases, similar results were obtained (Additional file 1: Table S1–S9).
Figure 3 shows the results comparing the risk of NODM between those taking pitavastatin and those taking atorvastatin + rosuvastatin before PSM across the 10 databases. The HR (95% CI) for the meta-analytic estimate of the risk of NODM showed that pitavastatin was associated with a lower risk of NODM than atorvastatin + rosuvastatin (HR 0.86; 95% CI 0.74–0.99). After PSM, results from three databases (KDH, EUMC, and NHIMC) showed that the pitavastatin group had a significantly lower cumulative incidence of NODM than the atorvastatin + rosuvastatin group (Fig. 4). After pooling results from 10 databases, the incidence rate of NODM during pitavastatin use was 21.7 per 1000 person-years and that of atorvastatin + rosuvastatin use was 24.2 per 1000 person-years. The meta-analysis of HR from 10 databases demonstrated that the pitavastatin group had a statistically significant lower risk of NODM than the atorvastatin + rosuvastatin group (HR 0.72; 95% CI 0.59–0.87) (Fig. 5).
In the sub-analysis, we created five 1:1 propensity-score-matched cohorts to compare pitavastatin vs. atorvastatin, pitavastatin vs. rosuvastatin, atorvastatin vs. rosuvastatin, pitavastatin vs. moderate-intensity atorvastatin + rosuvastatin, pitavastatin vs. moderate-intensity atorvastatin, pitavastatin vs. moderate-intensity rosuvastatin, and pitavastatin vs. high-intensity atorvastatin + rosuvastatin in the same manner. The meta-analysis of HR showed that pitavastatin was associated with a lower risk of NODM than atorvastatin or rosuvastatin (pitavastatin vs. atorvastatin: HR 0.69; 95% CI 0.54–0.88; pitavastatin vs. rosuvastatin: HR 0.74; 95% CI 0.55–0.99) (Fig. 6). However, no significant difference was observed in the risk of NODM between the atorvastatin and rosuvastatin groups (HR 1.08; 95% CI 0.90–1.29). In addition, pitavastatin was associated with a lower risk of NODM compared to moderate-intensity atorvastatin + rosuvastatin (HR 0.78; 0.62–0.98); however, no significant difference was observed between pitavastatin and high-intensity atorvastatin + rosuvastatin (HR 0.78; 95% CI 0.55–1.12) (Fig. 7). A consistently low risk of NODM in pitavastatin users were observed when compared with each of moderate-intensity atorvastatin or rosuvastatin users (pitavastatin vs. moderate-intensity atorvastatin: HR 0.79; 95% CI 0.64–0.97; pitavastatin vs. moderate-intensity rosuvastatin: HR 0.73; 95% CI 0.54–0.99) (Fig. 8).
Discussion
This is the first distributed network research focusing on NODM risk related to statins by head-to-head comparison using real-world clinical CDM data from 10 institutions. The present study showed that pitavastatin reduced the HR of NODM by 28% compared with atorvastatin + rosuvastatin. In sub-analysis, pitavastatin also had a significantly lower risk of NODM than each of atorvastatin or rosuvastatin. This effect was prominent in the comparison between pitavastatin and moderate-intensity atorvastatin or rosuvastatin, but there was no statistically significant difference in the comparison between pitavastatin and high-intensity atorvastatin or rosuvastatin. It is generally accepted that the benefits of statins for preventing cardiovascular disease far outweigh the risk of NODM; nevertheless, administration of statins with a lower risk of NODM is ideal for those at high-risk of diabetes [4, 27].
Several clinical trials and meta-analyses have revealed the effect of statins on NODM [3, 4]. The mechanism by which statins affect glucose homeostasis is not fully understood; however, several preclinical studies have demonstrated that statins may influence insulin signaling in peripheral tissues, resistance, and secretion, as well as pancreatic beta-cell function, and glucose metabolism [28, 29]. In contrast, there have been several experimental studies supplying evidence of a lower risk of NODM with pitavastatin. In an in vitro study, the reduction in the rate of insulin secretion in pancreatic islet β-cells treated with pitavastatin was less than that of those treated with atorvastatin or rosuvastatin, and cell viability was also better than that with other statins [28, 30]. Similarly, the reduction in coenzyme Q10 levels caused by statin treatment affects insulin secretion and abnormal glucose metabolism, but there is evidence that pitavastatin has minimal effects on coenzyme Q10 through its unique pharmacological mechanism [28, 31]. Moreover, the glucose uptake rate of human skeletal muscle after treatment with pitavastatin was better than that with atorvastatin or rosuvastatin [30]. In addition, adiponectin concentration is inversely correlated with insulin resistance and visceral obesity [32]. It has been reported that pitavastatin significantly increases adiponectin levels, while atorvastatin has neural effects on adiponectin levels and rosuvastatin has harmful effects. These findings could explain the lower risk of NODM after pitavastatin treatment [33, 34].
Clinical studies have suggested that pitavastatin may be associated with a lower risk of NODM than other statins. A network meta-analysis of 29 randomized clinical trials by Thakker et al. reported that pitavastatin had the lowest risk of NODM among six statins (rosuvastatin, atorvastatin, pravastatin, simvastatin, lovastatin, and pitavastatin) compared to the placebo; however, there was no significant difference between pitavastatin and atorvastatin or rosuvastatin [16]. Moreover, in a real-world cohort study of Asian patients, pitavastatin tended to have a lower HR for NODM than other statins, though the difference was not statistically significant [7]. A recently reported single-center study also showed that atorvastatin and rosuvastatin had a significantly higher risk of NODM than pitavastatin [35]. Nevertheless, there is a counterargument for these results. Cho et al. performed a single-center, retrospective study of 3680 patients and reported that pitavastatin had the highest HR for NODM than other statins with simvastatin as a reference [17]. As such, previous results from clinical studies are controversial, and data for the direct comparison of the risk of diabetes between pitavastatin and other statins is limited.
The present study is a meta-analysis of the results from CDM-converted multicenter EMR data analysis performed in large-scale PS matched cohorts. It has the advantage of reducing the risk of confounding bias and providing high-level evidence for the direct comparison of the risk of diabetes among the study drugs. Moreover, the present study was conducted on patients who had an observation period of ≥ 1 year before statin administration with no previous exposure to any statins. The active-comparator and new-user designs implemented in the present study could mitigate the methodological limitations of observational studies, such as immortal-time bias [36]. Moreover, to remove protopathic bias, NODM was defined as patients diagnosed at least 180 days (lag period) after the index date. Another strength of the present study was that it excluded patients who had serum HbA1c levels ≥ 5.7% at any point before and within 180 days after enrollment, which increased the reliability of the causal relationship between statins and NODM. In addition, we performed common statistical analysis on the databases using the same analytic R code, and the results of the study had relatively good reproducibility across all 10 databases. Future research could be expanded to large datasets.
Pitavastatin was classified as a moderate-intensity statin, and Choi et al. reported that it is associated with a lower incidence of NODM in patients with acute myocardial infarction compared to moderate-intensity atorvastatin or rosuvastatin [37]. The present study also showed that pitavastatin had a lower risk of NODM than moderate-intensity atorvastatin or rosuvastatin. However, the difference in NODM risk between pitavastatin and high-intensity atorvastatin or rosuvastatin did not reach statistical significance in the present study. The numerically highest difference in the incidence rate of NODM was observed between the pitavastatin and high-intensity atorvastatin or rosuvastatin groups (incidence rate of 21.0 vs. 28.6 per 1000 person-years, respectively). In general, high-intensity statins are associated with a greater risk of NODM than low-to-moderate-intensity statins [4]. Considering the wide range of confidence intervals, a possible explanation for our null finding is the relatively small sample size related to statistical power. Because high-intensity statins were often used following moderate-intensity statin therapy in routine clinical practice, there were a few patients who were new-user of high-intensity atorvastatin or rosuvastatin. In addition, the indications vary according to statin intensity, there was a disparity in the distribution of propensity scores between the pitavastatin and high-intensity atorvastatin or rosuvastatin groups. In this study, only 4416 patients were assigned to each group after 1:1 PSM, which was deemed the main reason for the lack of statistical significance between pitavastatin and high-intensity atorvastatin or rosuvastatin.
Since comparison of active treatment with placebo inevitably induces substantial bias in observational setting, we did not evaluate the risk of NODM in pitavastatin uses compared to non-statin users [36]. Some studies have shown that pitavastatin did not adversely affect HbA1c level [13, 38]. Recent retrospective study using the Korean insurance claims database reported that the risk of NODM was the largest for atorvastatin followed by rosuvastatin among individual statins when compared to non-statin users, but pitavastatin was not associated with increasing risk of NODM [39]. However, only 27 patients prescribed with pitavastatin were enrolled in that study. Another retrospective study showed that pitavastatin had a lower HR for NODM than atorvastatin or rosuvastatin, but which was significantly higher than that of the non-statin user group [40]. Therefore, further research is needed to investigate the risk of NODM in pitavastatin users compared to non-statin users.
Our study has several limitations. First, owing to the observational nature of the study design, residual confounding factors may have influenced the study results despite adjustment with large-scale propensity score matching. Moreover, some information regarding patients receiving antidiabetic drugs or statins at hospitals other than those included in the study may have been missed. Second, the definition of NODM differs from recent guidelines [41]. Since we could not confirm fasting blood glucose or two-hour plasma glucose during an oral glucose tolerance test, we used ICD-10 code, HbA1c, and diabetic drug history as criteria for NODM. Third, this study used an aggregate meta-analysis approach without pooling individual data. While our combined data sources provided the population size needed to demonstrate a statistically significant effect in favor of pitavastatin, only three databases had significant results separately. However, a previous study showed that aggregate meta-analysis yields estimate that are at least as precise and accurate as the pooled individual dataset [42].
Conclusions
In conclusion, pitavastatin was associated with a lower risk of NODM than atorvastatin or rosuvastatin in patients who were newly treated with statins. This study could guide the selection of statins for patients with a high risk of diabetes in clinical practice.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- NODM:
-
New-onset diabetes mellitus
- HR:
-
Hazard ratio
- CDM:
-
Common data model
- HbA1c:
-
Hemoglobin A1c
- ICD:
-
International classification of disease
- PSM:
-
Propensity score matching
References
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177–232.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742.
Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–64.
Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
Betteridge DJ, Carmena R. The diabetogenic action of statins—mechanisms and clinical implications. Nat Rev Endocrinol. 2016;12(2):99–110.
Yoon D, Sheen SS, Lee S, Choi YJ, Park RW, Lim HS. Statins and risk for new-onset diabetes mellitus. A real-world cohort study using a clinical research database. Medicine. 2016;95(46):e5429.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–30.
Alagona Jr P Pitavastatin: evidence for its place in treatment of hypercholesterolemia. Core Evid. 2010;5:91–105.
Abe M, Ozaki Y, Takahashi H, Ishii M, Masunaga N, Ismail TF, et al. Relation of renal function to mid-term prognosis of stable angina patients with high- or low-dose pitavastatin treatment: REAL-CAD substudy. Am Heart J. 2021;240:89–100.
Yoshida M, Nakamura K, Miyoshi T, Yoshida M, Kondo M, Akazawa K et al. Combination therapy with pemafibrate (K-877) and pitavastatin improves vascular endothelial dysfunction in dahl/salt-sensitive rats fed a high-salt and high-fat diet. Cardiovasc Diabetol. 2020;19(1):1–10.
Ihm S-H, Chung W-B, Lee J-M, Hwang B-H, Yoo K-D, Her S-H, et al. Efficacy and tolerability of pitavastatin versus pitavastatin/fenofibrate in high-risk Korean patients with mixed dyslipidemia: a multicenter, randomized, double-blinded, parallel, therapeutic confirmatory clinical trial. Clin Ther. 2020;42(10):2021–35.
Yokote K, Shimano H, Urashima M, Teramoto T Efficacy and safety of pitavastatin in Japanese patients with hypercholesterolemia: LIVES study and subanalysis. Expert Rev Cardiovasc Ther. 2011;9(5):555–62.
Gumprecht J, Gosho M, Budinski D, Hounslow N Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab. 2011;13(11):1047–55.
Vallejo-Vaz AJ, Seshasai SRK, Kurogi K, Michishita I, Nozue T, Sugiyama S, et al. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241(2):409–18.
Thakker D, Nair S, Pagada A, Jamdade V. Malik A Statin use and the risk of develo** diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25(10):1131–49.
Cho Y, Choe E, Lee YH, Seo JW, Choi Y, Yun Y et al. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism. 2015;64(4):482–8.
Park J, You SC, Cho J, Park CH, Shin WG, Park RW et al. Comparative risk of incidence and clinical outcomes of COVID-19 among proton pump inhibitor and histamine-2 receptor antagonist short-term users: a nationwide retrospective cohort study. BMC Pharmacol Toxicol. 2022;23(1):1–10.
Seo SI, Park CH, You SC, Kim JY, Lee KJ, Kim J et al. Association between proton pump inhibitor use and gastric cancer: a population-based cohort study using two different types of nationwide databases in Korea. Gut. 2021;70(11):2066–75.
Seo SI, You SC, Park CH, Kim TJ, Ko YS, Kim Y, et al. Comparative risk of Clostridium difficile infection between proton pump inhibitors and histamine-2 receptor antagonists: a 15‐year hospital cohort study using a common data model. J Gastroenterol Hepatol. 2020;35(8):1325–30.
Yoon D, Ahn EK, Park MY, Cho SY, Ryan P, Schuemie MJ et al. Conversion and data quality assessment of electronic health record data at a Korean tertiary teaching hospital to a common data model for distributed network research. Healthc Inform Res. 2016;22(1):54–8.
Makadia R, Ryan PB Transforming the Premier Perspective® hospital database into the Observational Medical Outcomes Partnership (OMOP) common data model. EGEMS. 2014;2(1):15.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285-350.
Schuemie MJ, Suchard M, Ryan P. CohortMethod: new-user cohort method with large scale propensity and outcome models. 2018. https://ohdsi.github.io/CohortMethod/.
Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23(1):1–17.
Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9.
Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60(14):1231–8.
Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63(6):735–45.
Koh KK, Lim S, Sakuma I, Quon MJ. Caveats to aggressive lowering of lipids by specific statins. Int J Cardiol. 2012;154(2):97–101.
Zhao W, Zhao SP. Different effects of statins on induction of diabetes mellitus: an experimental study. Drug Des Devel Ther. 2015;9:6211–23.
Kawashiri MA, Nohara A, Tada H, Mori M, Tsuchida M, Katsuda S et al. Comparison of effects of pitavastatin and atorvastatin on plasma coenzyme Q10 in heterozygous familial hypercholesterolemia: results from a crossover study. Clin Pharmacol Ther. 2008;83(5):731–9.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88.
Nomura S, Shouzu A, Omoto S, Inami N, Tanaka A, Nanba M et al. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb Res. 2008;122(1):39–45.
Chrusciel P, Sahebkar A, Rembek-Wieliczko M, Serban MC, Ursoniu S, Mikhailidis DP, et al. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208.
Liu WT, Lin C, Tsai MC, Cheng CC, Chen SJ, Liou JT, et al. Effects of pitavastatin, atorvastatin, and rosuvastatin on the risk of new-onset diabetes mellitus: a single-center cohort study. Biomedicines. 2020;8(11):499.
Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–41.
Choi JY, Choi CU, Hwang SY, Choi BG, Jang WY, Kim DY, et al. Effect of pitavastatin compared with atorvastatin and rosuvastatin on new-onset diabetes mellitus in patients with acute myocardial infarction. Am J Cardiol. 2018;122(6):922–8.
Huang CH, Huang YY, Hsu BR. Pitavastatin improves glycated hemoglobin in patients with poorly controlled type 2 diabetes. J Diabetes Investig. 2016;7(5):769–76.
Na E, Cho S, Kim DJ, Choi J, Han E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: a retrospective cohort study. Cardiovasc Diabetol. 2020;19(1):1–11.
Lee J, Noh Y, Shin S, Lim HS, Park RW, Bae SK et al. Impact of statins on risk of new onset diabetes mellitus: a population-based cohort study using the Korean National Health Insurance claims database. Ther Clin Risk Manag. 2016;12:1533–43.
Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Supplement_1):S17–38.
Lin D-Y, Zeng D On the relative efficiency of using summary statistics versus individual-level data in meta-analysis. Biometrika. 2010;97(2):321–32.
Acknowledgements
We acknowledge data custodians of Wonkwang University Hospital, Daegu Catholic University Medical Center, Myongji Hospital and Kangwon National University Hospital for supplying the CDM data.
Funding
This research was supported by the Hallym University Research Fund 2021 (HURF-2021-22) and a Grant (22213MFDS486) from Ministry of Food and Drug Safety in 2022.
Author information
Authors and Affiliations
Contributions
All authors contributed to literature review, study design, and data collection and interpretation. WWS and SEK contributed to data analysis and manuscript writing. SIS, YK, JJY, WGS, JK, SCY, RWP, YMP, KJK, SYR, MP, and ESJ provided critical opinions on the study design and manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Kangdong Sacred Hospital (IRB number 2019-03-008) and Ewha Womans University Mokdong Hospital (IRB number 2020-09-026). The IRB waived written informed consent and approved this study. The other eight hospitals are affiliated with the Research Border Free Zone of Korea CDM data network, which recognizes IRB approval of the research organizing center and waives the need for individual IRB approval. This study complied with the principles of the Declaration of Helsinki.
Consent for publication
The manuscript was approved by all authors for publication.
Competing interests
The authors declare that there are no relationships or activities that might bias or be perceived to bias their work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in KHNMC cohort. Table S2. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in WKUH cohort. Table S3. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in DCMC cohort. Table S4. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in AUMC cohort. Table S5. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in PNUH cohort. Table S6. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in EUMC cohort. Table S7. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in NHIMC cohort. Table S8. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in MJH cohort. Table S9. Baseline characteristics of patients with pitavastatin vs. atorvastatin or rosuvastatin in KWMC cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Seo, WW., Seo, S.I., Kim, Y. et al. Impact of pitavastatin on new-onset diabetes mellitus compared to atorvastatin and rosuvastatin: a distributed network analysis of 10 real-world databases. Cardiovasc Diabetol 21, 82 (2022). https://doi.org/10.1186/s12933-022-01524-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01524-6