Abstract
Radiation pneumonia (RP) is a common adverse reaction to radiation therapy in patients with chest tumors. Recent studies have shown that diabetes mellitus (DM), which can cause systemic multisystem damage, specifically targets lungs, and the incidence of RP in patients with a history of diabetes is higher than that in other patients with tumors who have undergone radiotherapy. DM is an important risk factor for RP in tumor patients undergoing RT, and patients with DM should be treated with caution. This article reviews research on the clinical aspects, as well as the mechanism, of the effects of diabetes on RP and suggests future research needed to reduce RP.
Similar content being viewed by others
Diabetes mellitus (DM) is a relatively common chronic metabolic disease [1]. The annual incidence of complications due to diabetes is increasing [2, 3]. Recent research has shown that diabetes is an important risk factor for radiation toxicity following radiotherapy for several tumors. Radiation-induced gastrointestinal and urogenital toxicity is more common in patients with DM [4, 5]. Radiation damage due to breast cancer treatment is also more common in patients with DM than in those without it [6].
Radiation therapy is a routine treatment for cancer after surgery. Radiation injury is one of the main adverse effects in patients who have undergone radiotherapy, and it is also the main limiting factor of the treatment [7]. When it occurs, radiation pneumonia (RP) usually presents within 1 to 6 months after completion of radiotherapy [8], with an incidence between 15 and 45% [4]. Patients with diabetes have shown a higher risk of radiation-induced damage [9], and many studies have indicated that lung tissue is a target for diabetes damage [10, 11], with an association between DM and RP. Glucocorticoids are the main drugs used to treat RP, but the administration of hormones can adversely affect blood glucose control and the insulin dosage required for diabetic patients. It also directly affects treatment of RP [12]. Therefore, some medical institutions exclude patients with severe diabetes from treatment with conventional radiation therapy [13]. The occurrence of RP in patients with breast tumors and DM has become a clinical concern. This article will review how DM affects RP based on clinical reports and mechanistic studies.
Clinical evidence that DM affects RP
Hsia et al. [14] retrospectively analyzed clinical data from 118 patients with lung cancer and reported that DM itself was not associated with a higher risk of RP. Similarly, Orton et al. evaluated the correlation between DM and RP in 66 lung cancer patients and reported that, although patients who had lung cancer and DM showed a higher incidence of RP than patients with lung cancer alone, this incidence was not significantly different from that in patients without DM [9]. However, both of these studies were reported as abstracts only; full reports were unavailable. In China, there is a number of literature indicating that DM is an important risk factor for RP [15,16,17]. However, most of these studies were published in Chinese, and were performed with a small sample size and limited research scope. Therefore, their generalizability is uncertain. Kalman's team performed 424 computed tomography follow-up scans at 3, 6, and 12 months after treatment of 116 patients, including 27 diabetic patients, that received stereotactic radiation therapy (SBRT), and found that, in diabetic patients, tissue has denser and hazier than that of the non-diabetic patients, and that this was most obvious at 3 months after treatment. This indicated that lung injury after pulmonary SBRT is related to DM and most obvious at early stages after treatment [18]. This may be the first report to show an increase in the severity of RP after SBRT in diabetic patients. Due to the small sample size of the study, the period of lung injury should be interpreted cautiously. However, similar findings on the necessary caution of SBRT for diabetic patients had be reported. Kong et al. evaluated by univariate and multivariate analyses the effects of DM and DM-related serum factors (HbA1c and FBG) on the development of RP in 123 lung cancer patients that underwent chest radiotherapy, and confirmed that DM, HbA1c, and fasting blood glucose were significantly associated with the development of RP above grade 3. This was the first published report on the impact of DM-related serum factors on the development of RP [19]. This clinical evidence suggests that people with DM are more likely to develop RP than other people with lung cancer, and similar to the report by Kalman et al., that radiotherapy and post-treatment monitoring of lung cancer patients with DM should be conducted with caution. Collecting the retrospective data of 268 patients with NSCLC after radiotherapy and chemotherapy, Sefika et al. evaluated the relationship between RP risk and potential predictors through univariate and multivariate analysis, concluding that DM is an independent risk factor for RP [20]. The study identified diabetes as an independent risk factor for RP. However, there is a lack of research on the effect of diabetes classification on RP. At the same time, more large-scale randomized controlled trials (RCTs) are gain further relevant clinical evidence of the interactions between DM and SBRT.
Mechanism underlying the effects of DM on RP
Promotion of an inflammatory response
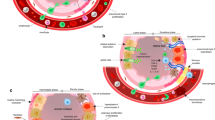
A 1993 article first described the relationship between tumor necrosis factor (TNF), inflammation, and insulin resistance in the journal Science [21]. At present, DM is considered a chronic inflammatory disease. Levels of inflammatory factors such as interleukin 6 (IL-6) and TNF-α are significantly increased in patients with DM, and type 2 DM (T2DM) is classified as a metabolic inflammatory disease [22, 23].
In the hyperglycemic environment of diabetic patients, anti-inflammatory cytokines such as IL-4, IL-5, and IL-13, which are typically maintained homeostatically in a microenvironment, lead to changes in the number and type of present cells [24, 25], presenting and releasing pro-inflammatory cytokines leading to chronic inflammation [26]. DM can also cause nuclear factor kappa-B (NF-κB) activation through receptor for advanced glycation endproducts (RAGE) [27], which is a member of the immunoglobulin superfamily of cell surface molecules, promoting TNF-α, IL-6, and IL-1β gene transcription and inflammation (Fig. 1) in the fat, muscle, and other tissues in our bodies [28]. Many reports have confirmed the important roles inflammatory cytokines such as IL-6, IL-8, and TNF-α play in mediating peripheral airway inflammation [29]. The "cytokine cascade" is currently recognized as the basis for RP pathogenesis [30]. In the early stages of radiation damage, changes in local inflammation and cytokine production can stimulate collagen synthesis in fibroblasts, leading to the development of pneumonia [31]. A hyperglycemic state activates inflammatory cytokines, aggravating lung tissue inflammation [32]. DM and its role in increasing inflammatory factors and exacerbating RP is gradually being recognized. Co-treatment of DM and RP should be the focus of future research (Fig. 2).
Immune dysregulation
In healthy individuals, immune responses are temporary. However, obesity, insulin resistance, persistent hyperglycemia, and other conditions can cause a chronic pathological immune imbalance that contributes to various diseases [33]. T2DM is an autoimmune disease, the accumulation of a large number of metabolites may cause immune dysfunction and damage immune function [34]. Changes in the proportions of T lymphocyte subsets can reflect changes in the body's immune status and are also closely related to the type and the degree of infection [35]. For example, the percentage of CD3 + and CD4 + T cells is lower in diabetic patients than in non-diabetics [36]. In addition, the hyperglycemic state of diabetic patients affects B lymphocyte function and humoral immunity, and results in IgM, IgA, and IgG antibody levels that indicate humoral immune deficiency [37]. T2DM can also impair NK cell function and increase the risk of infection and cancer [38, 39]. Immune imbalance is an important mechanism underlying infections concurrent with DM, because DM reduces a body’s resistance, and the higher incidence of vascular and peripheral neuropathy in diabetic patients [40]. Immune imbalance is also recognized in RP [41]. The immune system plays a role not only in the formation and development of RP but also prediction of this condition [42]. Diabetic patients have a disorder of glucose, fat, and protein metabolism, and the acidic environment in diabetic patients’ tissues reduces patients' cellular immune function, ultimately affecting the lungs [43] (Fig. 3).
Oxidative stress
In DM, glucose and its metabolites react with hydrogen peroxide in the presence of iron and copper ions, forming hydroxyl groups during auto-oxidation and generating a large amount of reactive oxygen species (ROS). ROS, in turn, promote the development of complications, such as hyperlipidemia, end-stage renal disease and apoptosis of islet cells [44]. Insulin resistance caused by chronic hyperglycemia is also thought to be related to the induction of oxidative stress [45]. Akash [46] et al. studied the level of 8-hydroxydeoxyribonucleic acid-modified protein in Goto Kakizaki rats, a model of T2DM, and showed that hyperglycemia is the main underlying factor of pancreatic β-cell oxidative stress, the type of stress caused by glucose that underlies glucotoxicity. In 309 diabetic patients vs. non-diabetic controls, Charles Sturt et al. observed significantly increased levels of glycosylated hemoglobin, lipids, and oxidative stress biomarkers, and suggested that these findings support a link between long-term hyperglycemia in T2DM and hyperglycemia-induced oxidative stress [47]. Chronic persistent hyperglycemia has also been reported to contribute to microvascular and macrovascular complications that occur through a series of mechanisms involving oxidative stress and inflammation [47]. Oxidative stress injury is involved in initiating RP. The "cytokine cascade theory [48]" promotes the idea that ROS damages lung parenchymal cells, causing these cells to secrete a large amount of inflammatory cytokines, chemokines, profibrotic cytokines, and other factors that cause RP. In addition, Ma [49] et al. found that, with increased production of oxygen free radicals, the activity of oxidative stress-sensitive kinases and transcription cytokines in macrophages increased rapidly, leading to the production of large numbers of inflammatory cytokine such as TNF-α, IL-6, and transforming growth factor-β (TGF-β) (Fig. 4).
Microvascular endothelial injury
As an important endocrine organ, the vascular endothelium produces cytokines can regulate vascular tension, vascular wall inflammation, and smooth muscle cell proliferation and adhesion, which can lead to vascular disease [50]. Recently, vascular endothelial injury has been recognized as a common cause of various DM vascular lesions [51], particularly at their initiation [52]. Animal experiments have shown that the basement membrane of alveolar epithelial and capillary endothelial cells in diabetic rats is significantly thickened, and some rats showed focal nodular changes that were very similar to the nodular changes in diabetic glomerular basement membranes [53]. Sustained hyperglycemia can also chronically damage vascular structures by promoting apoptosis of vascular endothelial cells [54], increasing the permeability of these cells [55] and regulating endothelial cell gene expression [56], eventually leading abnormal microvascular structure and function. Clinical studies have shown that in T2DM patients, the expression of some molecules that cause chronic structural damage to the vascular endothelium such as inflammatory factors (e.g., IL-6), vascular endothelial growth factor (VEGF), and angiopoietin (Ang) are upregulated, whereas the expression of molecules that exert vascular protective effects is significantly downregulated [57, 58]. The function and state of the endothelium depends not only on the extent of damage, but also on its ability to repair endogenously. In this regard, circulating endothelial progenitor cells (EPCs) play an important role because they can activate endothelial recovery [59]. In patients with diabetes, EPCs have been shown reduced ability to proliferate, adhere, and be incorporated into blood vessels [60]. In summary, DM causes endothelial dysfunction in many ways, but chronic damage to the structures and functions of vascular endothelial cells may be one of the main mechanisms underlying the development or aggravation of RP.
Microvascular occlusion
Microcirculation is the most basic structural and functional unit in the blood circulation system. It regulates vasomotor smooth muscle contraction and affects blood flow through nerves and Bodily fluids. Microvascular occlusive lesions are a general term for microcirculation duct, blood flow, and functional obstructive lesions. They mostly occur in pathological conditions such as metabolic disorders and inflammatory reactions. They are characterized by slowed microvascular blood flow, increased vascular permeability, endothelial cell damage, and leukocyte adhesion and migration [61]. Lungs are extremely rich in microvasculature. Microvascular endothelial cell damage and increased vascular permeability caused by DM may lead to thickening of the fused basal lamina of alveolar septal epithelial cells and alveolar capillary endothelial cell basal lamina. In addition, some studies have found that diabetic microangiopathy is related to an imbalance in the fibrinolytic system [62]. Tissue-type plasminogenactivator (t-PA) is the initiating factor for the plasmin system [63]. It is regulated in the blood by plasminogen activator inhibitor type 1 (PAI-1). Patients with DM have long-term hyperglycemia, which causes an increase in PAI-1 that reduces the synthesis and secretion of t-PA, causing disorders of the fibrinolytic and coagulation systems, platelet activation, and microthrombosis, implicating peripheral nerve tissues and blood vessels corresponding tissue and organ lesions [64]. DM can cause microvascular occlusive lesions that lead to microcirculation disturbances in the lungs, which limit dissipation of local inflammatory reactions [65,66,67].
Discussion
In the 1970s, Schuyler et al. [68] first proposed that lungs were a target organ of DM. In the past 40 years, evidence has accumulated that shows lung injury caused or exacerbated by high glucose can result in chronic progressive effects in lungs, poor quality of life, and limitations on the use of hormone therapies [69, 70]. The Third National Health and Nutrition Survey in the United Kingdom showed that lung function declines with impaired glucose tolerance [71], and that the relationship between T2DM and lung-related damage was significant [72].Through meta-analysis, ** radiotherapy plans and determining radiation doses. Maintaining fasting blood glucose levels within the normal range can reduce the occurrence of RP in patients with tumors and DM [79, 80]. However, there is still much that needs to be explored in interactions between DM and radiotherapy.
This article has several limitations. First, clinical studies reported in this article are mostly retrospective; therefore, there may be inherent biases. Specifically, we do not know how patients with DM faired long after treatment, with the potential for development of late-onset RP. Second, the sample sizes of these studies were relatively small. Third, patients and their tumors are heterogeneous. Despite these shortcomings, we believe that this article explores some important unresolved issues concerning the relationship between DM and RP.
Conclusion
DM is an important risk factor for RP in tumor patients undergoing RT, and patients with DM should be treated with caution when undergoing this therapy.
Availability of data and materials
The datasets generated and/or analyzed in the current study are not publicly available due to state restrictions. Thus, information may compromise research participant privacy/consent but data are available from the corresponding author on reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- RP:
-
Radiation pneumonia
- RCTs:
-
Randomized controlled trials
- TNF:
-
Tumor necrosis factor
- NF-κB:
-
Nuclear factor kappa-B
- RAGE:
-
Receptor for advanced glycation endproducts
- EPCs:
-
Endothelial progenitor cells
- PAI-1:
-
Plasminogen activator inhibitor type 1
References
Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12(6):357–70.
Lutz SZ, Staiger H, Fritsche A, et al. Antihyperglycaemic therapies and cancer risk. Diabetes Vasc Dis Res. 2014;11(6):371–89.
Wu D, Hu D, Chen H, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–41.
Zaorsky NG, Shaikh T, Ruth K, et al. Prostate cancer patients with unmanaged diabetes or receiving insulin experience inferior outcomes and toxicities after treatment with radiation therapy. Clin Genitourin Cancer. 2017;15(2):326–35, e323.
Alashkham A, Paterson C, Hubbard S, Nabi G. What is the impact of diabetes mellitus on radiation induced acute proctitis after radical radiotherapy for adenocarcinoma prostate? A prospective longitudinal study. Clin Transl Radiat Oncol. 2019;14:59–63.
Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–6.
Bledsoe TJ, Nath SK, Decker RH, et al. Radiation pneumonitis. Clin Chest Med. 2017;38(2):201–8.
Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–7.
Orton MD, Mukhopadhyay ND, Weiss E. Evaluation of diabetes as a risk factor for the development of clinically symptomatic pneumonitis following Stereotactic Body Radiation Therapy (SBRT). Int J Radiat Oncol Biol Phys. 2013;87(2):S514.
Popov D, Simionescu M. Alterations of lung structure in ex-perimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur Respir J. 1997;10(18):1850–8.
Lin CC, Chang CT, Li TC, et al. Objective evidence of impair of alveolar integrity in patients with non-insulin-dependent mellitus using radionuclide inhalation lung scan. Lung. 2002;180(3):181–6.
Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–74.
Yamashita H, Takahashi W, Haga A, et al. Radiation pneumonitis after stereotactic radiation therapy for lung cancer. World J Radiol. 2014;9(6):708–15.
Hsia WJ, Jan N, Mukhopadhyay ND, Weiss E. Effect of diabetes mellitus and selected medications on the development of radiation pneumonitis in patients with locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;93(3):403.
Luo W, Wang Y, Tang SM. Effect of different fractionated radiotherapy modes on radiation pneumonitis after radical mastectomy in patients with diabetes mellitus. J Modern Oncol. 2019;16(27):2879–82.
Guo F, Du AN, Shi JL, et al. Clinical study of radiation pneumonitis in patients with esophageal cancer and diabetes after intensity-modulated radiotherapy. Chin J Radiol Health. 2018;06(27):628–30.
Zhang X-J, Sun J-G, Sun J, et al. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138(12):2103–16.
Kalman Noah S, Hugo Geoffrey D, Mahon Rebecca N, et al. Diabetes mellitus and radiation induced lung injury after thoracic stereotactic body radiotherapy. Radiother Oncol. 2018;129(2):270–6.
Kong M, Lim YJ, Kim Y, et al. Diabetes mellitus is a predictive factor for radiation pneumonitis after thoracic radiotherapy in patients with lung cancer. Cancer Manag Res. 2019;11:7103–10.
Ergen SA, Dincbas FO, Yücel B, et al. Risk factors of radiation pneumonitis in patients with NSCLC treated with concomitant chemoradiotherapy––Are we underestimating diabetes?––Turkish oncology group (TOG)/Lung cancer study group. 2020;14(9):871–879.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
Stolecki D. Markers of the progression of complications in patients with type 2 diabetes: a one-year longitudinal study. Exp Clin Endocrinol Diabetes. 2014;122(08):484–90.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–74.
Gunasekaran MK, Virama-Latchoumy AL, Girard AC, et al. TLR4-dependant pro-inflammatory effects of HMGB1on human adipocyte. Adipocyte. 2016;5(4):384.
Ferrante AW Jr. Macrophages, fat, and the emergence of immunometabolism. J Clin Invest. 2013;123(12):4992–3.
Beijnum JRV, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11(1):91–9.
Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group BOX 1 protein. Am J Physiol. 2003;284(4):870–9.
Rubinsztajn R, Przybyowski T, Paplińska-Goryca M, et al. Correlation between hyperinflation defined as an elevated RV/TLC ratio and body composition and cytokine profile in patients with chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2015;83(2):120–5.
Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys. 2004;58(02):463–9.
Büttner C, Skupin A, Reimann T, et al. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17(3):315–25.
Rinne ST, Feemster LC, Collins BF, et al. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014;10(1):1–6.
Tanaka SI, Isoda F, Ishihara Y, et al. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol. 2001;54(3):347–54.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Kim JH, Cho TS, Moon JH, et al. Serial changes in serum eosinophil-associated mediators between atopic and non-atopic children after mycoplasma pneumoniae pneumonia. Allergy Asthma Immunol Res. 2014;6(5):428–33.
He T, Tao J, Wang X, et al. Effects of cisatracurium in combination with ventilation on inflammatory factors and immune variations in sepsis rats. Exp Ther Med. 2018;15(5):4414–8.
Chen YH, Lee CH, Hsu TH, et al. Submerged-culture mycelia and broth of the maitake medicinal mushroom grifola frondosa (higher basidiomycetes) alleviate type 2 diabetes-induced alterations in immunocytic function. Int J Med Mushrooms. 2015;17(6):541–56.
Cheekatla SS, Tripathi D, Venkatasubramanian S, et al. NK-CD11c+ cell crosstalk in diabetes enhances IL-6-mediated inflammation during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12(10):e1005972.
PiTkiewicz P, Bernat-Karpińska M, et al. NK cell count and glucotransporter 4 (GLUT4) expression in subjects with type 2 diabetes and colon cancer. Diabetol Metab Syndr. 2016;8(1):38.
Naruishi K, Omori K, Maeda H, et al. Immune responses to porphyromonas gingivalis infection suppress systemic inflammatory response in experimental murine model. J Biol Regul Homeost Agents. 2011;25(2):195–202.
Park HR, Jo SK. Lasting effects of an impairment of Th1-like immune response inγ-irradiated mice:a resemblance between irradiated mice and aged mice. Cell Immunol. 2011;267(1):1–8.
Tsoutsou PG. The interplay between radiation and the immune system in the field of post-radical pneumonitis and fibrosis and why it is important to understand it. Expert Opin Pharmacother. 2014;15(13):1781–3.
Sinclair AJ, Conroy SP, Bayer AJ. Impact of diabetes on physical function in older people. Diabetes Care. 2008;31(2):233–5.
Browne JL, Hooshmand S, Elam M, et al. The relationship between inflammation, oxidative stress, and metabolic risk factors in type 2 diabetic patients. Phytomed Int J Phytother Phytopharmacol. 2012;20(6):470–80.
Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation-a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581(19):3734–42.
Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked?: Oxidative stress and diabetes mellitus. J Cell Biochem. 2017;118(11):3577–85.
Butkowski EG, Jelinek HF. Hyperglycaemia, oxidative stress and inflammation markers. Redox Rep. 2017;22:257–64.
Novakova-Jiresova A, Luijk PV, Goor HV, et al. Changes in expression of injury after irradiation of increasing volumes in rat lung. Int J Radiat Oncol Biol Phys. 2007;67(5):1510–8.
Ma ZC, Hong Q, Wang YG, et al. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol Pharm Bull. 2010;33(1):29–34.
Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation. 2007;115(10):1285–95.
Xu F, Liu Y, Zhu X, et al. Protective effects and mechanisms of vaccarin on vascular endothelial dysfunction in diabetic angiopathy. Int J Mol Sci. 2019;20(18):4587–602.
Chen RF, Chang CH, Wang CT, et al. Suppression of oxygen radicals protects diabetic endothelium damage and tissue perfusion in a streptozotocin-induced diabetes rodent mode. Ann Plas Surg. 2019;82(1S):18–22.
Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–62.
Basmaeil YS, Al Subayyil AM, Khatlani T, et al. Human chorionic villous mesenchymal stem/stromal cells protect endothelial cells from injury induced by high level of glucose. Stem Cell Res Ther. 2018;9(1):238–57.
Tonade D, Liu H, Palczewski K, Kern TS. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia. 2017;60(10):2111–20.
Irhimeh MR, Hamed M, Barthelmes D, et al. Identification of novel diabetes impaired miRNA-transcription factor co-regulatory networks in bone marrow-derived Lin-/VEGF-R2+ endothelial progenitor cells. PLoS ONE. 2018;13(7):e0200194.
Falkevall A, Mehlem A, Palombo I, et al. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 2017;25(3):713–26.
Cui X, Chopp M, Zacharek A, et al. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43(1):285–92.
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–6.
Tomaiuolo G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics. 2014;8(5):89–110.
Chan K, Chen S, Chen P. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J Funct Foods. 2019;53:22–7.
Opdenakker G, El-Asrar AA. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell Mol Life Sci. 2019;76:3157–66.
Suzanne M. The full spectrum of Alzheimer’s disease is rooted in metabolic derangements that drive type 3 diabetes. Adv Exp Med Biol. 2019;1128:45–83.
Long J, He CJ, Ding H, et al. Effect of shock wave on vascular lesions in diabetic rats. Pain Phys. 2019;22(5):505–10.
Lee S, Lee MY, Nam JS, Kang S, Park JS, Shin S, Ahn CW, Kim KR. Hemorheological approach for early detection of chronic kidney disease and diabetic nephropathy in type 2 diabetes. Diabetes Technol Ther. 2015;17(11):808–15.
Domingueti CP, Dusse LM, Carvalho M, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat. 2016;30(4):738–45.
Schuyler M, Niewoehner D, Inkley S, et al. Abnormal lung elasticity in juvenile diabetes mellitus. Am Rev Respir Dis. 1976;113(1):37–41.
ALIMO. Pulmonarycomplications in diabetes mellitus. Mymensingh Med J. 2014; 23(3):603–605.
Mekov EV, Slavova YG, Genova MP, et al. Diabetes mellitus type 2 in hospitalized COPD patients: impact on quality of life and lung function. Folia Med. 2016;58(1):36–41.
Mckeever TM, Weston PJ, Hubbard R, et al. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161(6):546–56.
Ford ES, Mannino DM, National H, et al. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care. 2004;27(12):2966–70.
Pinnix C, Perkins GH, Strom EA, et al. Topical hyaluronic acid vs standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: single-blind randomized phase III clinical trial. Int J Radiat Oncol Biol Phys. 2012;83(4):1089–94.
Plama DA, Senan S, Tsu**o K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87(4):690–6.
Malenica M, Šilar M, Dujic T, et al. Importance of inflammatory markers and IL-6 for diagnosis and follow up of patients with type 2 diabetes mellitus. Med Glas Ljek Komore Zenicko-doboj Kantona. 2017;14(2):169–75.
Wu HP, Chu CM, Lin CY, et al. Liver cirrhosis and diabetes mellitus are risk factors for staphylococcus aureus infection in patients with healthcare-associated or hospital-acquired pneumonia. Pulm Med. 2016;2016:4706150.
Walter RE, Beiser A, Giveller RJ, et al. Association between glycemic state and hung function. Am Respir Crit Care Med. 2003;167(5):911.
Niren K, Shah PD, Wasim E, et al. A novel sodiumglucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2012;32(1):80–94.
Bernard L, Reix N, Benabu JC, et al. Breast cancer and diabetes mellitus: complex interactions. Gynecol Obstet Fertil. 2016;44(12):701–11.
Huang YJ, Huang TW, Lin FH, et al. Radiation therapy for invasive breast cancer increases the risk of second primary lung cancer: a nationwide population-based cohort analysis. J Thorac Oncol. 2017;12(5):782–90.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This article was funded by the Chinese National Health and Family Planning Commission Health Bureau Central Health Research Project (W2017BJ43).
Author information
Authors and Affiliations
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, G., Li, Y., Zhao, Q. et al. Effects of diabetes on the development of radiation pneumonitis. Respir Res 22, 160 (2021). https://doi.org/10.1186/s12931-021-01754-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01754-4