Abstract
Background
Treatment protocols for two-stage revision arthroplasty with diabetes mellitus (DM) have not yet been established. The control of glycated hemoglobin (HbA1c) in two-stage revision arthroplasty is still debated. This study aimed to clarify the importance of preoperative HbA1c levels before each stage of revision arthroplasty and to analyze the risk factors for reinfection.
Methods
Five hundred eighty-eight patients suffered from first-time PJI and was treated in our institute from January 1994 to December 2010 were reviewed. The mean follow-up time was 13.8 (range, 10.2–24.8) years. Patients underwent two-stage revision arthroplasty with DM at presentation were included. The endpoint of the study was reinfection of the revision arthroplasty. Demographic, survivorship, and surgical variables were also analyzed.
Results
Eighty-eight patients were identified and grouped by HbA1c level before the first stage surgery: Groups 1 and 2 had HbA1c levels < 7% and ≥ 7%, respectively. Reinfection was identified in 4.55% (2/44) and 18.18% (8/44) of the patients in Groups 1 and 2, respectively. Survivorship analysis revealed correction of the HbA1c before the final stage of revision arthroplasty as an independent factor (p < 0.001). The identified risks for reinfection were HbA1c levels ≥ 7% before final-stage surgery, ≥ 3 stages of revision arthroplasty, and extended-spectrum beta-lactamase (ESBL)-Escherichia coli PJI.
Conclusion
The HbA1c level before the final stage of revision arthroplasty could affect staged revision arthroplasty outcomes. Therefore, the necessity of postponing the elective final-stage revision arthroplasty procedure for HbA1c control should be further investigated in the future.
Similar content being viewed by others
Background
Periprosthetic joint infection (PJI) is one of the most devastating complications following total joint arthroplasty (TJA) [1]. It has become the second main reason for revision arthroplasty, in both knee and hip arthroplasty [2]. The number of TJAs has increased recently with the evolution of arthroplasty; however, the PJI rate remains fairly constant without improvement [3]. Nonetheless, the socioeconomic costs associated with PJIs are extremely burdensome to the healthcare system. These costs are driven by comorbidities associated with repeated surgeries and the high mortalities of up to 21.12% [4]. Consequently, efforts have been made to improve treatment outcomes, with increasing research focused on PJI treatment over the last decade [5].
PJI treatment has evolved over the years [5]. The gold standard treatment for chronic PJI is two-stage revision arthroplasty. During the first stage, antibiotic-loaded bone cement (ALBC) is implanted as a spacer, following debridement with implant removal. The final-stage procedure will be scheduled at a later time after the convalescence of infection [6]. Nevertheless, the success rates for these treatment policies vary, ranging from 66 to 95% for two-stage revision arthroplasty, and are far from optimal [7]. Two-stage revision arthroplasty outcomes are well documented; however, to the best of our knowledge, the real situation and principles for patients with DM are still unclear.
The worldwide prevalence of diabetes among adults was 9.3% in 2019, and it is predicted to reach 10.2% by 2030 [8]. Meanwhile, the rate of arthroplasty in patients with DM in the USA has also been projected to increase to approximately 8% annually by 2030 [9]. Most studies have shown that inadequate perioperative glycemic control is highly associated with surgical site infection (SSI) or PJI after TJA [10]. HbA1c reflects the average plasma glucose concentration. However, controversy exists on the relationship between elevated serum HbA1c levels and SSI or PJI following TJA despite a well-known risk factor, DM [11]. Nevertheless, the American Diabetes Association (ADA) guidelines recommend an HbA1c threshold lower than 7% (53 mmol/mol) to decrease postoperative complications [12]. However, the threshold remains contentious in the orthopedics field, not only for TJA [13], but especially for two-stage revision arthroplasty. Only one study indicated that higher postoperative glucose variability during reimplantation surgery increased the risk of treatment failure [14]. Therefore, this study aimed to (1) evaluate the association between preoperative HbA1c levels at each stage of surgery and adverse outcomes following two-stage revision arthroplasty in patients with DM and (2) analyze the risk factors for reinfection of the revision arthroplasty in these patients from different viewpoints (including host, bacteria, surgery, and DM medication).
Methods
Database
This retrospective cohort study collected PJI patients (hip or knee) who were treated in our institute from January 1994 to December 2010. From January 1994, the database was built and these patients were followed at least ten years with institutional review board approval (IRB: 201601034B0). We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 996.66 to search these patients in hospital database. The cohort was checked by two independent orthopedic assistants. We excluded infection after fracture reduction and fixation, primary septic arthritis or synchronous PJI.
Study sample
All PJIs following a primary hip or knee prosthesis in the database with a follow-up ≥ 10 years were investigated (n = 588). Only two-stage revision arthroplasty for treating first PJI were included (n = 421). We excluded one-stage revision arthroplasty (n = 45), amputation (n = 5), fusions (n = 6), and debridement, antibiotics and implant retention (DAIR)(n = 111) for the treatment of first PJI.
Study design
Patients with DM experienced a PJI episode was identified in our database, and further analyzed the HbA1c level: the ADA guidelines recommend an HbA1c threshold of 7% [12] before the first stage revision arthroplasty. The patients were dichotomized into Groups 1 and 2 with HbA1c levels < 7% and ≥ 7%, respectively. Outcomes and risk factors leading to recurrent PJIs were analyzed in each group. The endpoint of the study was defined as reinfection, which was on fulfillment of the criteria of PJI mentioned later, after the staged revision surgery.
Patient demographics, comorbidities, characteristic of the previous arthroplasty, and revision arthroplasty before the PJI (if applicable), procedures to manage the PJI, and causative pathogens for PJI episodes were all recorded and analyzed. Patients who had unclear chart records, were followed for less than ten years, or did not follow the DM and PJI treatment protocols in our institute were excluded.
Definition of terms
PJI was defined and assigned if the 2011 MSIS criteria were fulfilled [15] and was treated according to the protocols of our institute with Tsukayama’s classification [16]. If the symptoms were present for > 4 weeks, it was considered a late chronic infection. In such cases, the gold standard treatment is staged revision arthroplasty. An interval with implantation of an antibiotic-loaded bone cement spacer provides a higher success rate.
In our institute, staged revision arthroplasty proceeded according to a strict protocol following a diagnosis of late chronic infection, summarized as follows: (1) On initial presentation of PJI, if patients had systemic inflammatory response syndrome (SIRS), two sets of blood cultures were taken. (2) During the first stage of staged revision arthroplasty, we obtained multiple intraoperative cultures (three sets) with radical debridement. The prosthesis was removed and the ALBC was implanted. (3) Postoperatively, 4 weeks of systemic intravenous (IV) plus 2 weeks of oral antimicrobial agents were administered according to the culture result, on advice from an infectious disease specialist. (4) Six weeks were spent on medication “holiday.” (5) After a 3-month (in total) interim with convalescence of the PJI, joint arthrocentesis was performed. (6) Once recovery from infection was confirmed, the final staged revision arthroplasty was performed. However, if negative culture result was encountered, we used empirical antibiotics with vancomycin and ceftazidime according to our previous common bacteria report in our institute [17].
Not all PJIs resolve after two-stage revision arthroplasty; some require multiple stages. Staged revision arthroplasty was defined by the number of surgeries performed prior to final reimplantation (three-stage or four-stage revision arthroplasty). The microorganism profiles were analyzed during all PJI episodes, and polymicrobial PJI was diagnosed when more than one single species of microorganism was identified.
Treatment protocols for patients with HbA1c ≥ 7%
We controlled blood sugar levels according to the guidelines published by ADA [12]. We evaluated the patients’ underlying disease, daily schedule, eating and exercise habits, and drug adherence, and prescribed the most suitable anti-diabetic medications. We also seriously considered body weight changes and the risk of hypoglycemia. Insulin would be suggested if the HbA1c level was over 10% or there were already four different kinds of anti-diabetic drugs other than insulin. The target of sugar control varied from person to person based on the patient’s age, comorbidities, life span, and self-care function.
Statistical analysis
Continuous variables were compared using analysis of variance if showing a normal distribution and the relationship of qualitative variables was evaluated using the chi-square or Fisher’s exact test. The risk factors contributing to treatment failure (recurrent PJI) was evaluated using univariate and multivariate logistic regression models. For all tests, p < 0.05 was considered significant. Processing and data analysis were performed using International Business Machines Corporation (IBM) Statistical Product and Service Solutions (SPSS), statistics for Windows, version 20.0 (IBM Corp., Armonk, N.Y., USA).
Results
Eighty-eight patients with DM who were diagnosed with PJI were identified. These were followed retrospectively for more than ten years (range, 10.2–24.8 years) after the first-stage surgery. There was no case suffered from death during the follow-up in this cohort. These patients were further dichotomized into Groups 1 and 2 according to HbA1c level: 7% at the initial presentation of PJI (Group 1 < 7%; Group 2 ≥ 7%). There were forty-four patients in each group during the study period.
Demographic data were compared between groups (Table 1). DM medications were also analyzed between the groups (Table 2). Risk factors contributing to reinfection were an HbA1c level ≥ 7% at the final-stage surgery, ≥ 3 stages of resection arthroplasty, and the presence of ESBL-producing Escherichia coli (Table 3).
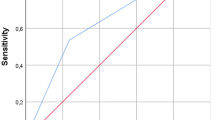
Reinfection of the revision arthroplasty was identified in 4.55% (2/44) and 18.18% (8/44) of the patients in Groups 1 and 2, respectively, without significant differences in survival curves (p = 0.15). In Group 2, there were ten patients who followed our treatment protocol for DM and PJI and still had HbA1c levels ≥ 7% before the elective final-stage revision arthroplasty procedure. The reinfection rate in this subgroup was 80% (4/5). The Kaplan–Meier survivorship curves were analyzed by group, with a cut-off HbA1c level of 7%. Figure 1 shows the groups according to HbA1c level during the first-stage surgery for the initial PJI. Figure 2 shows groups according to HbA1c level during the final-stage procedure. The endpoint was an episode of recurrent PJI.
Discussion
To the best of our knowledge, this is the first study to explore the role of HbA1c in staged revision arthroplasty. We found that the survivorship of staged revision arthroplasty in DM patients was favorable in those who had well controlled HbA1c levels (within 7%) before the final-stage surgery. The identified risks for reinfection after staged revision arthroplasty were HbA1c levels ≥ 7% before final-stage surgery, ≥ 3 stages of revision arthroplasty, and extended-spectrum beta-lactamase (ESBL)-Escherichia coli PJI.
Many studies have revealed the obscure role of HbA1c in TJA. In 2012, Richard et al. discussed DM, HbA1c, and TJA [18]. PJIs in TJA have increased in patients with DM, regardless of HbA1c level. In 2015, Hilal et al. published the results of a large database cohort study and demonstrated a significant PJI risk in patients with DM diagnoses, under DM medication, and those with perioperative hyperglycemia. However, the HbA1c level was not a risk factor for PJI. Nevertheless, other studies and guidelines have shown the importance of HbA1c; therefore, HbA1c should also be monitored (6.5–7.5%) before TJA [12]. Moreover, Jordan et al. tried in vain to identify the threshold HbA1c level in TJA [19]. In summary, the incidence of PJIs might increase; however, there is no good independent predictor of PJI after TJA.
Wang et al. reported that DM patients with increased postoperative glucose variability after two-stage revision arthroplasty had a higher chance of reinfection after revision surgery [14]. However, this association was more robust in patients without diabetes. In our cohort, patients with DM who had suffered a first PJI could be further divided into two groups based on HbA1c levels. However, an important question is whether HbA1c levels should be strictly controlled before the elective final-stage revision arthroplasty procedure. We directly compared the outcomes among DM patients with different first presentations. Unexpectedly, the division in our cohort was equal during the follow-up. The patients followed the same staged revision arthroplasty and DM treatment protocols and were attended by endocrinologists at the same institute. In summary, regardless of the patient’s HbA1c level during the first stage of revision arthroplasty, the real strategy of consequence is to control the HbA1c level to within 7% before the final stage of revision arthroplasty.
Furthermore, we investigated whether the DM medication in our series was related to reinfection rate. Table 2 summarizes the baseline DM medication during the first stage of revision arthroplasty. Although there were no significant differences in the duration of DM between the groups, the fasting glucose level before the first stage of revision arthroplasty was higher in Group 2. Moreover, endocrinologists’ prescriptions showed a higher chance of using sulfonylurea, dipeptidyl peptidase 4 (DPP4) inhibitor, acarbose, and insulin combined with an oral anti-diabetic agent to control DM at the initial presentation during the first stage of revision arthroplasty. According to the literature, the risk of PJI increased among patients receiving DM medication, but was not associated with the type of medication [20]. We cannot draw any conclusion on whether prescribed DM medication is a risk factor for reinfection; however, we have revealed that endocrinologists made great efforts to maintain HbA1c levels within the normal range in patients in Group 2. Nonetheless, there were still five patients with HbA1c levels ≥ 7% despite following our DM protocols. This is called “refractory DM.” Refractory DM could be defined as HbA1c levels ≥ 9% despite specialist care for at least six months [21]. It accounts for 15% of DM patients with some identified risk factors, such as early age of onset, number of diabetes education program visits, number of oral therapies, and insulin use [22]. This retrospective study aimed to establish whether further postponement of elective surgery on account of the HbA1c level is warranted in the future in patients under the PJI treatment protocol in our institute. A literature review revealed that surgery may help functionality and mobility, which increase activity and exercise. This indicates that postponing surgery may increase the long-term risk of DM complications [23].
In our study, undergoing ≥ 3 stages of revision arthroplasty was an independent risk factor for reinfection of the revision arthroplasty. Following the protocols in our institute, patients underwent reoperation after the first-stage surgery with repeat extensive synovectomy and debridement. Multiple surgeries may lead to contracture or scarring of the soft tissue, which might decrease susceptibility to antibiotics due to the hypovascular status [24]. A vicious cycle of resistant bacterial wound be developed with treatment failure and reinfection.
As far as this vicious cycle is concerned, ESBL-E. coli could be considered the second risk factor for recurrent PJI in our cohort. Tissue culture revealed significant differences only regarding ESBL-E. coli. Meanwhile, we also found that E. coli was significantly more frequently cultured in Group 2 than in Group 1. The prevalence of E. coli PJI is approximately 8% [25], and this resistant strain is highly related to poor outcomes in two-stage revision arthroplasty [26]. However, for DM patients with E. coli PJI, our study is the first to identify the relative risk. We found a higher risk of uncontrolled DM in patients with E. coli PJI, and ESBL-E. coli was an independent risk factor for reinfection after two-stage revision arthroplasty.
This study also had several limitations. First, possible missing data with selection bias might be encountered due to the retrospective case–control design. We try to minimize bias by telephone consultation of each patient followed by the same treatment protocols and rehabilitation programs. Second, we have limited cases due to the long-term follow-up. Third, we could not use the level of fructosamine in our institute yet, which could further represent the sugar levels over a 2 to 3 weeks period. This makes it a valuable marker for both screening and monitoring therapeutic interventions for TJA [27, 28]. However, the actual role in staged resection arthroplasty has not been well proven, we could investigate it in the future.
Conclusions
HbA1c levels before the final stage of revision arthroplasty could affect staged revision arthroplasty outcomes, especially for refractory DM patients. Patients who had ≥ 3 stages of revision arthroplasty or PJI caused by ESBL-E. coli were at a greater risk of recurrent PJI. Therefore, the necessity of postponing the elective final-stage revision arthroplasty procedure until patients’ HbA1c levels have been controlled should be further investigated in the future.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- HbA1c:
-
Glycated hemoglobin
- ESBL:
-
Extended-spectrum beta-lactamase
- PJI:
-
Periprosthetic joint infection
- TJA):
-
Total joint arthroplasty
- ALBC:
-
Antibiotic-loaded bone cement
- SSI:
-
Surgical site infection
- ADA:
-
American Diabetes Association
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- DAIR:
-
Debridement, antibiotics and implant retention
- ESR:
-
Erythrocyte sedimentation rate
- CRP:
-
C-reactive protein
- PMN%:
-
Neutrophil percentage
- SIRS:
-
Systemic inflammatory response syndrome
- IV:
-
Intravenous
- IBM:
-
International Business Machines Corporation
- SPSS:
-
Statistical Product and Service Solutions
- DPP4:
-
Dipeptidyl peptidase 4
References
Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplasty. 2017;32:2663–8. https://doi.org/10.1016/j.arth.2017.03.066.
Kamath AF, Ong KL, Lau E, Chan V, Vail TP, Rubash HE, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30:1492–7. https://doi.org/10.1016/j.arth.2015.03.035.
Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the medicare population. J Arthroplasty. 2018;33:3238–45. https://doi.org/10.1016/j.arth.2018.05.042.
Natsuhara KM, Shelton TJ, Meehan JP, Lum ZC. Mortality during total hip periprosthetic joint infection. J Arthroplasty. 2019;34:S337–42. https://doi.org/10.1016/j.arth.2018.12.024.
Xu C, Goswami K, Li WT, Tan TL, Yayac M, Wang SH, et al. Is Treatment of periprosthetic joint infection improving over time? J Arthroplasty. 2020;35:1696-702.e1. https://doi.org/10.1016/j.arth.2020.01.080.
Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472:2208–14. https://doi.org/10.1007/s11999-014-3571-4.
Tan TL, Goswami K, Fillingham YA, Shohat N, Rondon AJ, Parvizi J. Defining treatment success after 2-stage exchange arthroplasty for periprosthetic joint infection. J Arthroplasty. 2018;33:3541–6. https://doi.org/10.1016/j.arth.2018.06.015.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Yang L, Sun Y, Li G, Liu J. Is hemoglobin A1c and perioperative hyperglycemia predictive of periprosthetic joint infection following total joint arthroplasty?: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96: e8805. https://doi.org/10.1097/MD.0000000000008805.
Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2018;33:2312-21.e3. https://doi.org/10.1016/j.arth.2018.02.020.
Citak M, Toussaint B, Abdelaziz H, Klebig F, Dobinsky A, Gebauer M, et al. Elevated HbA1c is not a risk factor for wound complications following total joint arthroplasty: a prospective study. Hip Int. 2020;30:19–25. https://doi.org/10.1177/1120700020926986.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S66-76. https://doi.org/10.2337/dc20-S006.
Tarabichi M, Shohat N, Kheir MM, Adelani M, Brigati D, Kearns SM. Determining the threshold for HbA1c as a predictor for adverse outcomes after total joint arthroplasty: a multicenter, retrospective study. J Arthroplasty. 2017;32:S263-7.e1. https://doi.org/10.1016/j.arth.2017.04.065.
Wang SH, Xu C, Tan TL, Goswami K, Cooper AM, Parvizi J. Increased postoperative glucose variability is associated with adverse outcome following two-stage exchange arthroplasty for periprosthetic joint infection. J Arthroplasty. 2020;35:1368–73. https://doi.org/10.1016/j.arth.2019.11.046.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26:1136–8. https://doi.org/10.1016/j.arth.2011.09.026.
Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–23. https://doi.org/10.2106/00004623-199604000-00005.
Tsai YF, Chang CH, Lin YC, Lee SH, Hsieh PH, Chang YH. Different microbiological profiles between hip and knee prosthetic joint infections. J Orthop Surg. 2019;27(2):2309499019847768.
Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27:726-9.e1. https://doi.org/10.1016/j.arth.2011.09.013.
Cancienne JM, Werner BC, Browne JA. Is There an association between hemoglobin A1C and deep postoperative infection after TKA. Clin Orthop Relat Res. 2017;475:1642–9. https://doi.org/10.1007/s11999-017-5246-4.
Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94: e101. https://doi.org/10.2106/JBJS.J.01935.
Alharbi TJ, Tourkmani AM, Rsheed AB, Al Abood AF, Alotaibi YK. Sociodemographic and clinical predictors of refractory type 2 diabetes patients: findings from a case-control study. Saudi Med J. 2021;42:181–8.
Aronson R, Orzech N, Ye C, Goldenberg R, Brown V. Specialist-led diabetes registries and predictors of poor glycemic control in type 2 diabetes: insights into the functionally refractory patient from the LMC Diabetes Registry database. J Diabetes. 2016;8:76–85. https://doi.org/10.1111/1753-0407.12257.
Lopez LF, Reaven PD, Harman SM. Review: The relationship of hemoglobin A1c to postoperative surgical risk with an emphasis on joint replacement surgery. J Diabetes Complications. 2017;31:1710–8. https://doi.org/10.1016/j.jdiacomp.2017.08.016.
McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9:987–1007. https://doi.org/10.2217/fmb.14.64.
Kheir MM, Tan TL, Ackerman CT, Modi R, Foltz C, Parvizi J. Culturing periprosthetic joint infection: number of samples, growth duration, and organisms. J Arthroplasty. 2018;33:3531-6.e1. https://doi.org/10.1016/j.arth.2018.06.018.
da Silva RB, Salles MJ. Outcomes and risk factors in prosthetic joint infections by multidrug-resistant gram-negative bacteria: a retrospective cohort study. Antibiotics (Basel). 2021;10:340. https://doi.org/10.3390/antibiotics10030340.
Shohat N, Goswami K, Breckenridge L, Held MB, Malkani AL, Shah RP. Fructosamine is a valuable marker for glycemic control and predicting adverse outcomes following total hip arthroplasty: a prospective multi-institutional investigation. Sci Rep. 2021;11:2227. https://doi.org/10.1038/s41598-021-81803-6.
Shohat N, Tarabichi M, Tan TL, Goswami K, Kheir M, Malkani AL (2019) 2019 John Insall Award: Fructosamine is a better glycaemic marker compared with glycated haemoglobin (HbA1C) in predicting adverse outcomes following total knee arthroplasty. The Bone & Joint Journal 101-B: 3–9. https://doi.org/10.1302/0301-620X.101B7.BJJ-2018-1418.R1
Acknowledgements
We thank to Chun-Chieh Chen and Hsin-Nung Shih for their contributions of patients, and Uni-edit for editing and proofreading this manuscript.
Funding
One of the authors (Sheng‐Hsuan Lin) was supported by the Ministry of Science and Technology, Taiwan (MOST 108–2636-B-009 -001) for statistical analysis. One of the authors (Yu-Chih Lin) was supported by Chang Gung Memorial Hospital (CORPG5J0021, CORPG5G0021) for study design, monitoring, and interpretation.
Author information
Authors and Affiliations
Contributions
YC Lin participated in sequence alignment, the design of the study, and writing of the final manuscript. YH Lin participated in the field of endocrinology and the design of the study. JH Chou and YT Lo traced the medical records with complete follow-up. CH Chang and SH Lee performed the statistical analyses. SH Lin conceived the study, participated in its design and coordination, and helped assemble the draft manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study involved the comparison and analysis of de-identified, population-level data and adhered to the Declaration of Helsinki. Our study was approved by the Institutional Review Board (IRB: 201601034B0) at Chang Gung Memorial Hospital (CGMH) and the consent to participate was waived.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, YC., Lin, YH., Chou, JH. et al. Higher reinfection rate after two-stage revision arthroplasty in patients with refractory diabetes mellitus: a retrospective analysis with a minimum ten-year follow up. BMC Musculoskelet Disord 23, 990 (2022). https://doi.org/10.1186/s12891-022-05964-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05964-9