Abstract
Objective
A notable research gap exists in the systematic review and meta-analysis concerning the efficacy, immunogenicity, and safety of the respiratory syncytial virus (RSV) prefusion F vaccine.
Methods
We conducted a comprehensive search across PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov to retrieve articles related to the efficacy, immunogenicity, and safety of RSV prefusion F vaccines, published through September 8, 2023. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
A total of 22 randomized controlled trials involving 78,990 participants were included in this systematic review and meta-analysis. The RSV prefusion F vaccine exhibited a vaccine effectiveness of 68% (95% CI: 59–75%) against RSV-associated acute respiratory illness, 70% (95% CI: 60–77%) against medically attended RSV-associated lower respiratory tract illness, and 87% (95% CI: 71–94%) against medically attended severe RSV-associated lower respiratory tract illness. Common reported local adverse reactions following RSV prefusion F vaccination include pain, redness, and swelling at the injection site, and systemic reactions such as fatigue, headache, myalgia, arthralgia, nausea, and chills.
Conclusions
Our meta-analysis suggests that vaccines using the RSV prefusion F protein as antigen exhibit appears broadly acceptable efficacy, immunogenicity, and safety in the population. In particular, it provides high protective efficiency against severe RSV-associated lower respiratory tract disease.
Similar content being viewed by others
Introduction
Respiratory syncytial virus (RSV), discovered in 1956, is a negative-sense single-stranded RNA virus belonging to the Pneumonaviridae family. RSV is highly contagious and represents a major burden of respiratory disease worldwide, causing severe and even fatal respiratory infections and bronchiolitis, especially in the elderly (≥ 65 years), young children (< 5 years), and those with underlying chronic diseases (e.g., pulmonary and circulatory diseases) [1]. In 2019, globally, there were 33 million events of RSV-associated acute lower respiratory tract infection (uncertainty range, 2.54 to 446 million) and 1.01 million total RSV-attributable deaths (84 500 to 125 200) in young children [2].
There has been a long road with multiple obstacles to develo** a safe and effective RSV vaccine. Earlier vaccines provided insufficient protection as they used the post-F conformation as the vaccine antigen. This is because multiple unique antigenic sites are exposed on the surface of the F protein before RSV fuses with the host cell membrane. Following fusion, the F protein adopts a very different confirmation in which several antigenic sites are no longer exposed [3]. Thus, the stabilization of the pre-F conformation has made it possible to develop effective subunit vaccines [4]. On May 3, 2023, the U.S. Food and Drug Administration (FDA) approved the world’s first RSV vaccine (developed by GSK) and on May 31, 2023, the Pfizer vaccine, both for adults older than 60 years of age. Both vaccines use a prefusion stable variant of the F protein. RSV prefusion F vaccine has become a hot spot in the research of vaccines against RSV. A large number of clinical studies have investigated its protective efficacy. However, to date, no systematic reviews have been performed on the efficacy, immunogenicity and safety of RSV prefusion F vaccine. In this review, we compared the protective efficacy, antibody titer levels, and adverse reaction profiles of different RSV prefusion F vaccines between immunized individuals and controls.
Methods
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5].
Search strategy
In September 2023, in accordance with the study protocol, we conducted searches across several databases, including Medline via PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov, to identify articles published up to September 8, 2023. The following MeSH (Medical Subject Heading) terms and search terms were used: (“Respiratory Syncytial Viruses or RSV”) AND (“vaccine or vaccination or efficacy or adverse event”).
Eligibility criteria
The inclusion criteria included: (1) individual study populations being at least twenty cases; (2) the use of prefusion F protein as an immunogen is explicitly stated; (3) clinical trials in human subjects have been published. No language restrictions were imposed on the publications. To enhance the validity of the data, we excluded non-peer-reviewed articles from preprint databases.
Study selection
In this review, we employed a two-stage approach for screening, initially assessing titles and abstracts followed by full-text articles. Two researchers independently reviewed each title, abstract, and full text, with any discrepancies resolved through consensus with a third researcher. The efficacy of the vaccines were assessed on three endpoints. First, the efficacy of the vaccine in preventing RSV-associated acute respiratory illness which was defined as the ability of the vaccine to prevent RT-PCR-confirmed RSV infection within seven days of acute respiratory illness symptom onset. Second, the efficacy of the vaccine in preventing medically attended RSV-associated lower respiratory tract illness which was defined as at least two symptoms or signs of acute respiratory infection lasting at least 24 h (cough, abnormal breathing, fever, lethargy, or any other respiratory symptom of concern). Third, the efficacy of the vaccine in preventing medically attended severe RSV-associated lower respiratory tract illness which was defined as tachypnea (respiratory rate ≥ 70 breaths per minute in infants younger than two months [60 days] of age or ≥ 60 breaths per minute in those between two months and 12 months of age); SpO2 < 93% while the infant was breathing ambient air; use of oxygen delivered through a high-flow nasal cannula or mechanical ventilation; admission to an intensive care unit for more than 4 h; and unresponsiveness or unconsciousness. The efficacy of the RSV vaccine was based on assessing its efficacy during the first RSV season (about 6 months) after vaccination. All the efficacy endpoints were considered if they occurred at least seven days after the full regimen of the vaccine.
Data extraction
Two researchers extracted data using a predefined extraction form. Subsequently, all key extracted data underwent review and quality checking by the same two researchers at the conclusion of the data extraction phase. Data on study characteristics encompassed information regarding the setting, primary and secondary outcomes, study design, sample size, and exclusion and inclusion criteria. Participant data included details such as sex, age, and relevant medical history, including disease and treatment history. Intervention-related data consisted of the vaccine type and brand, dosing schedule, the number of participants receiving each type and brand of vaccine, and the median or mean interval between doses. Data pertaining to immunogenicity results included details such as the assay type, the specific antibody measured, T cell response, the method of measurement, intervals of sample collection, and the number of measurements conducted.
Risk of bias assessment
Two investigators independently evaluated the risk of bias in the included studies based on critical criteria, including random sequence generation, allocation concealment, blinding of participants, personnel, and outcomes, incomplete outcome data, selective outcome reporting, and other potential sources of bias, following the methods recommended by The Cochrane Collaboration. The risk of bias graph was generated using Revman 5.4 software. The following judgments were used: low risk, high risk, or unclear. Authors resolved disagreements by consensus and further article review if necessary.
Data analysis
We used RevMan 5.4 statistical software to pool dichotomous outcomes, with the risk ratio (RR) and its 95% confidence interval (CI) as the effect measures. RR < 1 implies a lower risk in the vaccinated group compared to the control group, and P < 0.05 indicates that this difference is statistically significant. The I2 statistic was used to estimate the level of heterogeneity, and significant heterogeneity was considered when the I2 value was > 50%. Vaccine efficacy was calculated using the fixed effects RR. This study applied the accepted statistical vaccine efficacy formula, (1 − RR) ×100, for calculating the pooled vaccine efficacy from the pooled RR. We conducted visual examinations of funnel plots and utilized Egger’s test to assess potential publication bias. Additionally, we employed the trim-and-fill analysis to evaluate the effect of publication bias on the pooled effect size estimates. Influence analysis, which constitutes a form of sensitivity analysis, was performed to identify the impact of individual studies on the combined estimates.
Results
Study selection and study characteristics
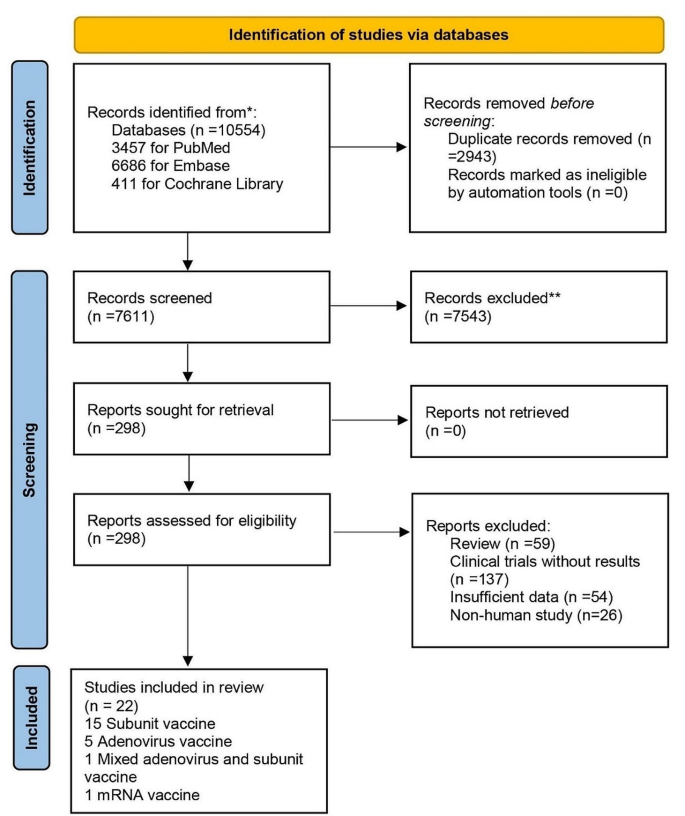
A total of 10,554 records were initially retrieved from the database. After screening titles and abstracts, we evaluated 298 full texts of potentially eligible reports; a total of 22 articles were included, involving 78,990 participants (Fig. 1) [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Of the 22 eligible studies, eight (36%) studies were analyzed to evaluate the efficacy of RSV prefusion F vaccines, 20 (91%) studies were analyzed to evaluate immunogenicity, and 22 (100%) studies were analyzed to evaluate safety (Table 1). The included studies reported data for four vaccine types: 15 (68%) for subunit vaccines, five (23%) for adenovirus vaccines, one (4%) for mixed adenovirus and subunit vaccines, and one (4%) for mRNA vaccines. The 22 included studies involved diverse populations, with 10 involving older adults over 60 years of age, 4 involving pregnant women, 3 involving non-pregnant women, and 7 involving healthy adults. The included studies involved more than 20 countries or regions, with 11 (50%) studies being multinational, six (27%) studies from Spain, followed by two (9%) studies from Australia, and one each from Japan, Canada, and the United Kingdom. 12 (55%) of the eligible studies were observer-blinded and 10 (45%) were double-blinded.
Risk of bias assessment of included studies
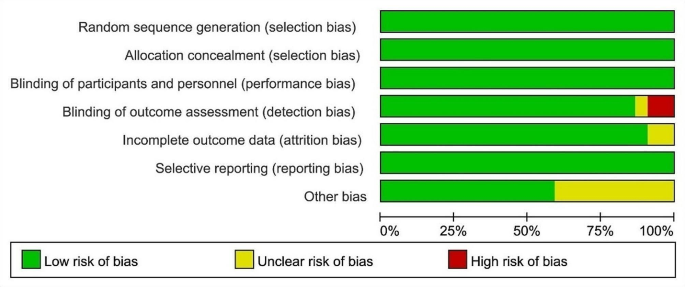
Twenty-two studies used Cochrane collaboration tools for independent risk of bias assessment, only two studies had high risk in blinding of outcome assessment, and most studies were low risk in all evaluated domains (Fig. 2). Overall, all of these included studies had a low risk of bias, with blinding and other biases in outcome assessment being the main risk factors.
Efficacy of RSV prefusion vaccine
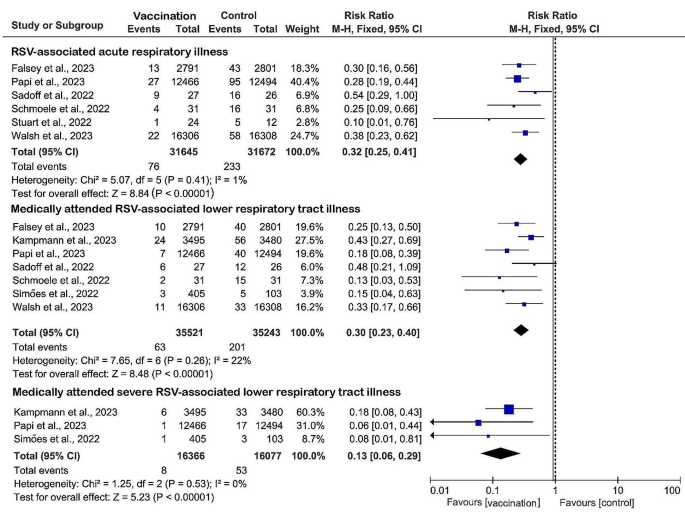
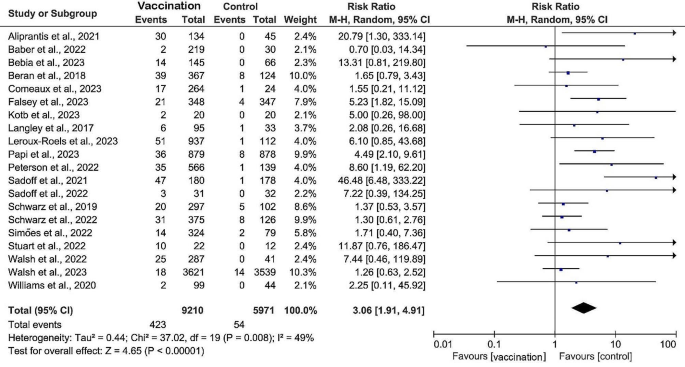
Six (27%) studies were included to evaluate the efficacy of RSV prefusion vaccine in the prevention of RSV-associated acute respiratory illness. Data from 31,645 vaccinated patients compared with 31,672 controls showed a significant pooled risk reduction in the vaccinated group, with a RR of 0.32 (95% CI: 0.25 to 0.41, I2 = 1%) and an overall vaccine efficacy of 68% (95% CI: 59–75%) (Fig. 3). A total of seven (32%) studies assessing the efficacy of vaccination against medically attended RSV-associated lower respiratory tract illness with data from 35,521 vaccinated versus 35,243 controls showed similarly significant pooled risk reductions in vaccinated groups, with a RR of 0.30 (RR 0.30, 95% CI: 0.23 to 0.40, I2 = 22%). Three (14%) studies reported the lowest RR (RR 0.13, 95% CI: 0.06 to 0.29, I2 = 0%) and minimal heterogeneity in severe RSV-associated lower respiratory tract illness requiring medical attention in the group that received the RSV prefusion F vaccines, with an overall vaccine efficacy of 87% (95% CI: 71–94%). When sensitivity analyses were performed, the heterogeneity of the pooled effects of the results did not change substantially after retaining only subunit vaccines, indicating that our results are robust and reliable.
Immunogenicity of RSV prefusion vaccine
Following the inclusion criteria, 20 studies (91%) on the immunogenicity of RSV prefusion F vaccines were included in this systematic review article (Table 2). There was a significant increase in neutralizing antibody titers against RSV-A in all studies, with a maximum increase of more than 20-fold from baseline. The neutralizing antibody titer against RSV-B was also significantly increased at about one month after immunization, with an increase of more than 1.4-fold compared with baseline. Seven studies examined T cell responses after vaccine immunization simultaneously, and the results showed that mixed adenovirus and subunit vaccine produced the strongest cellular immune responses, with up to 13-fold increase in interferon-γ secretion compared with baseline.
Safety of RSV prefusion vaccine
The safety profiles of 22 studies were reviewed, and adverse effects of RSV prefusion F vaccination included local reactions such as pain, redness, and swelling at the vaccination site and systemic reactions such as fatigue, headache, Myalgia, joint pain, nausea, and chills (Table 3). The subunit vaccine had the lowest risk of local and systemic adverse reactions, with RR of 2.79 (95% CI: 1.47 to 6.00, I2 = 77%) and 1.24 (95% CI: 0.95 to 1.63, I2 = 74%), respectively, and the risk of serious adverse events (grade ≥ 3) was also the lowest (RR 2.11, 95% CI: 1.41 to 3.15, I2 = 25%) (Fig. 4; Table 3). Redness was the predominant local reaction observed among recipients of the subunit vaccine (RR 4.77, 95% CI: 3.08 to 7.38, I2 = 41%). Conversely, pain at the injection site was the most common local symptom in the mRNA vaccine (RR 40.63, 95% CI: 5.85 to 282.44). Myalgia (RR 3.96, 95% CI: 2.35 to 6.66, I2 = 29%), nausea (RR 3.74, 95% CI: 0.83 to 16.9, I2 = 75%) and chill (RR 7.37, 95% CI: 4.20 to 12.94, I2 = 0%) were the most common symptoms reported in recipients of adenovirus vaccine. Of note, the mRNA vaccine exhibited the highest risk of adverse effects graded as 3 or higher (RR 20.79, 95% CI: 1.30 to 333.14). No RSV prefusion F vaccine-related deaths were recorded in these studies.
Discussion
In this systematic review and meta-analysis of 22 studies, we explore for the first time the efficacy, immunogenicity, and safety of RSV prefusion F vaccine. We found that administration of the RSV prefusion F vaccine significantly reduced the risk of RSV-associated acute respiratory illness, particularly the risk of severe cases of RSV-associated lower respiratory tract illness requiring medical attention. Previous studies have found that vaccines using the fused RSV F protein as antigen, although immunogenic, do not prevent RSV-associated acute respiratory illness in the elderly, and there is no clinically identifiable patient population that may benefit from this vaccine [28]. The failure of these clinical studies has led to intensive investigation of the immune mechanism of RSV. Valuable experience has been accumulated for the development of safe and effective vaccines targeting the F prefusion protein of RSV. In eight studies involving the evaluation of vaccine efficacy, subunit vaccines appeared to provide better protection than adenovirus vaccines, but due to the limited number of studies of the two vaccines included in this study, further research remains imperative.
This study provides a comprehensive assessment of the available literature on RSV prefusion F vaccines. We found that existing subunit vaccines, adenovirus vaccines, mixed subunit and adenovirus vaccines, and mRNA vaccines were able to generate significant immune responses against RSV in vaccine recipients. The titers of neutralizing antibodies against RSV-A and RSV-B and RSV-specific ligation antibodies were significantly different among different vaccine types due to the differences in immunogenicity composition, whether they contained adjuvants or not, immunization dose, immunization times, and detection time. In our study, five studies used the ELISPOT assay to measure T-cell immune responses and showed that subunit vaccines elicited weaker T-cell responses than adenovirus vaccines, mixed subunit and adenovirus vaccines, and mRNA vaccines, which is consistent with the results of a large number of studies of COVID-19 vaccines [29, 30].
Local adverse reactions after vaccination are more common than systemic adverse reactions. For different vaccine types, subunit vaccines are significantly safer and have lower incidence of local and systemic adverse reactions. Consistent with our results, the mRNA vaccine was associated with the highest incidence of adverse reactions except for a few [31]. In addition, mRNA vaccines have a higher association with serious adverse effects than other vaccine types [32]. Myalgia, nausea, and chills were the most common symptoms reported by adenovirus vaccine recipients, findings that were also similar to those previously reported for influenza and COVID-19 vaccines [30]. In theory, these differences could be attributed to differences in the strength of the immune response to the different vaccines [33, 34], as confirmed by the efficacy and immunization results of this review.
In addition, there is concern about whether RSV vaccination can cause a potentially risky rare neurologic disorder (Guillain-Barre syndrome). While GBS is considered uncommon, it remains a significant subject of discussion in the context of vaccination. Previous research on influenza vaccination has reported an eightfold rise in the risk of GBS [35]. Similarly, investigations into COVID-19 vaccines have indicated diverse clinical associations between COVID-19 vaccination and GBS [36, 37]. It is noteworthy that, reassuringly, there is currently no observed elevated risk of GBS associated with RSV vaccination.
This study has several limitations. First, current studies of RSV vaccine protection have been based on assessments of effectiveness during the first RSV season after vaccination (approximately 6 months). There were insufficient data to evaluate the duration of efficacy and immune effects after vaccination, and whether the vaccines result in long-term adverse events, thus necessitating long-term surveillance and study for the population. Second, the study included four vaccine types, but there was considerable variation in the number of studies across vaccine types. To eliminate this effect, we performed a subgroup analysis.
In conclusion, our meta-analysis suggests that vaccines using the RSV prefusion F protein as antigen exhibit favorable efficacy, immunogenicity, and safety in the population. In particular, it provides high protective efficiency against severe RSV-associated lower respiratory tract disease.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
References
Langedijk AC, Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol 2023.
Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EAF, Campbell H, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–8.
Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun. 2015;6:8143.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Aliprantis AO, Shaw CA, Griffin P, Farinola N, Railkar RA, Cao X, Liu W, Sachs JR, Swenson CJ, Lee H, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum Vaccines Immunotherapeutics. 2021;17(5):1248–61.
Baber J, Arya M, Moodley Y, Jaques A, Jiang Q, Swanson KA, Cooper D, Maddur MS, Loschko J, Gurtman A, et al. A phase 1/2 study of a respiratory Syncytial Virus Prefusion F Vaccine with and without adjuvant in healthy older adults. J Infect Dis. 2022;226(12):2054–63.
Bebia Z, Reyes O, Jeanfreau R, Kantele A, De Leon RG, Sánchez MG, Banooni P, Gardener GJ, Rasero JLB, Pardilla MBE, et al. Safety and Immunogenicity of an investigational respiratory Syncytial Virus Vaccine (RSVPreF3) in mothers and their infants: a phase 2 Randomized Trial. J Infect Dis. 2023;228(3):299–310.
Beran J, Lickliter JD, Schwarz TF, Johnson C, Chu L, Domachowske JB, Van Damme P, Withanage K, Fissette LA, David MP, et al. Safety and Immunogenicity of 3 formulations of an investigational respiratory Syncytial Virus Vaccine in Nonpregnant women: results from 2 phase 2 trials. J Infect Dis. 2018;217(10):1616–25.
Comeaux CA, Bart S, Bastian AR, Klyashtornyy V, De Paepe E, Omoruyi E, van der Fits L, van Heesbeen R, Heijnen E, Callendret B et al. Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.preF-based vaccine combinations: a Randomized, Double-blind, Placebo-Controlled, phase 1/2a study. J Infect Dis 2023.
Falsey AR, Williams K, Gymnopoulou E, Bart S, Ervin J, Bastian AR, Menten J, De Paepe E, Vandenberghe S, Chan EKH, et al. Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N Engl J Med. 2023;388(7):609–20.
Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, Baker J, Pérez Marc G, Radley D, Shittu E, et al. Bivalent Prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–64.
Kotb S, Haranaka M, Folschweiller N, Nakanwagi P, Verheust C, De Schrevel N, David MP, Mesaros N, Hulstrøm V. Safety and immunogenicity of a respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine in older Japanese adults: a phase I, randomized, observer-blind clinical trial. Respiratory Invest. 2023;61(2):261–9.
Langley JM, Aggarwal N, Toma A, Halperin SA, McNeil SA, Fissette L, Dewé W, Leyssen M, Toussaint JF, Dieussaert I. A Randomized, Controlled, Observer-Blinded phase 1 study of the Safety and Immunogenicity of a respiratory Syncytial Virus Vaccine with or without Alum Adjuvant. J Infect Dis. 2017;215(1):24–33.
Leroux-Roels I, Davis MG, Steenackers K, Essink B, Vandermeulen C, Fogarty C, Andrews CP, Kerwin E, David MP, Fissette L, et al. Safety and Immunogenicity of a respiratory Syncytial Virus Prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 Randomized Clinical Trial. J Infect Dis. 2023;227(6):761–72.
Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, Schwarz TF, van Zyl-Smit RN, Campora L, Dezutter N, et al. Respiratory Syncytial Virus Prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608.
Peterson JT, Zareba AM, Fitz-Patrick D, Essink BJ, Scott DA, Swanson KA, Chelani D, Radley D, Cooper D, Jansen KU, et al. Safety and Immunogenicity of a respiratory Syncytial Virus Prefusion F Vaccine when Coadministered with a Tetanus, Diphtheria, and Acellular Pertussis Vaccine. J Infect Dis. 2022;225(12):2077–86.
Sadoff J, De Paepe E, DeVincenzo J, Gymnopoulou E, Menten J, Murray B, Rosemary Bastian A, Vandebosch A, Haazen W, Noulin N, et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J Infect Dis. 2022;226(3):396–406.
Sadoff J, De Paepe E, Haazen W, Omoruyi E, Bastian AR, Comeaux C, Heijnen E, Strout C, Schuitemaker H, Callendret B. Safety and Immunogenicity of the Ad26.RSV.preF investigational vaccine Coadministered with an Influenza Vaccine in older adults. J Infect Dis. 2021;223(4):699–708.
Schmoele-Thoma B, Zareba AM, Jiang Q, Maddur MS, Danaf R, Mann A, Eze K, Fok-Seang J, Kabir G, Catchpole A, et al. Vaccine efficacy in adults in a respiratory Syncytial Virus Challenge Study. N Engl J Med. 2022;386(25):2377–86.
Schwarz TF, Johnson C, Grigat C, Apter D, Csonka P, Lindblad N, Nguyen TL, Gao FF, Qian H, Tullio AN, et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a Randomized Trial in Nonpregnant Women. J Infect Dis. 2022;225(12):2067–76.
Schwarz TF, McPhee RA, Launay O, Leroux-Roels G, Talli J, Picciolato M, Gao F, Cai R, Nguyen TL, Dieussaert I, et al. Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in Nonpregnant women: a phase 2, Randomized Trial. J Infect Dis. 2019;220(11):1816–25.
Simões EAF, Center KJ, Tita ATN, Swanson KA, Radley D, Houghton J, McGrory SB, Gomme E, Anderson M, Roberts JP, et al. Prefusion F protein-based respiratory Syncytial Virus immunization in pregnancy. N Engl J Med. 2022;386(17):1615–26.
Stuart A, Virta M, Williams K, Seppa I, Hartvickson R, Greenland M, Omoruyi E, Bastian AR, Haazen W, Salisch N et al. Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector RSV Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV Seropositive Children 12–24 Months. Journal of infectious diseases 2022.
Walsh EE, Falsey AR, Scott DA, Gurtman A, Zareba AM, Jansen KU, Gruber WC, Dormitzer PR, Swanson KA, Radley D, et al. A Randomized Phase 1/2 study of a respiratory Syncytial Virus Prefusion F Vaccine. J Infect Dis. 2022;225(8):1357–66.
Walsh EE, Pérez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, Polack FP, Llapur C, Doreski PA, Ilangovan K, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465–77.
Williams K, Bastian AR, Feldman RA, Omoruyi E, de Paepe E, Hendriks J, van Zeeburg H, Godeaux O, Langedijk JPM, Schuitemaker H, et al. Phase 1 safety and immunogenicity study of a respiratory Syncytial Virus Vaccine with an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in adults aged ≥ 60 years. J Infect Dis. 2020;222(6):979–88.
Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Takas T, Levin MJ, Falsey AR. An Adjuvanted, Postfusion F protein-based vaccine did not prevent respiratory Syncytial Virus illness in older adults. J Infect Dis. 2017;216(11):1362–70.
Al-Sheboul SA, Brown B, Shboul Y, Fricke I, Imarogbe C, Alzoubi KH. An immunological review of SARS-CoV-2 infection and vaccine serology: innate and adaptive responses to mRNA, Adenovirus, inactivated and protein subunit vaccines. Vaccines 2022;11(1).
McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6(1):74.
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021;93(12):6486–95.
Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, Shaban D, Al Khames Aga LA, Agha MYR, Traqchi M. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588–94.
Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, Wu X, Liu J, Zhao D, Li Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther. 2022;7(1):94.
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100.
Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, Eddins DL, Bryan JA. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105–23.
Chowdhury S, Chowdhury S. Association of Guillain-Barré syndrome following COVID-19 vaccination. Int J ImmunoPathol Pharmacol. 2023;37:3946320231199349.
Abara WE, Gee J, Marquez P, Woo J, Myers TR, DeSantis A, Baumblatt JAG, Woo EJ, Thompson D, Nair N, et al. Reports of Guillain-Barré Syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845.
Acknowledgements
We also thanks for the support of Construction of Key Clinical Specialties in Guangxi (Guiweiyifa [2022]-NO.21) and the Key Laboratory of Molecular Pathology of Guangxi.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Y.P. and H.L. analyzed the data, and drafted and revised the manuscript. X.Z., D.C., Q.L. and F.T. retrieved and collected the data. X.L. and Y.L. supervised the study and approved the final manuscript. All authors have agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pang, Y., Lu, H., Cao, D. et al. Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis. BMC Public Health 24, 1244 (2024). https://doi.org/10.1186/s12889-024-18748-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-18748-8