Abstract
Background
Liver cancer is ranked fifth in incidence and second in mortality among cancers in Taiwan. Nevertheless, the Taiwan government does not screen for liver cancer in its free cancer screening and preventive health examination service. This study compared the differences in cancer stage and survival between patients who received an initial liver cancer diagnosis in outpatient departments (OPDs) and those who received such a diagnosis in emergency departments (EDs).
Methods
This retrospective cohort study used the 2000–2016 National Health Insurance Database to obtain a sample from 2 million Taiwanese residents. To evaluate the effect of the utilization of the adult health examination offered to people aged ≥ 40 years, patients aged ≥ 40 years who received an initial liver cancer diagnosis between 2003 and 2015 were followed up until December 31, 2016.
Results
In total, 2,881 patients were included in this study. A greater proportion of cancer cases in the OPD group were non-advanced than those in the ED group (75.26% vs. 54.23%). Having stage C or D cancer, having a low monthly salary, and a Charlson comorbidity index score ≥ 8, not having hepatitis B, being divorced, and attending a non-public hospital as the primary care institution were risk factors for initial ED diagnosis. The risk of liver cancer-specific death among the ED group patients was 1.38 times that among the OPD group patients (adjusted hazard ratio = 1.38, 95% confidence interval [CI] = 1.14–1.68, P < 0.001). However, the use of health examination did not exert a significant effect on the likelihood of liver cancer diagnosis in an ED (adjusted odds ratio = 0.86, 95% CI = 0.61–1.21, P = 0.381).
Conclusion
Government-subsidized health examinations are insufficient to prevent first-ever diagnosed liver cancers in EDs. Patients with liver cancers diagnosed in EDs had a higher risk of advanced stage and mortality. For early detection and treatment, the government may consider implementing liver cancer screening for high-risk and low-socioeconomic people.
Similar content being viewed by others
Background
Liver cancer is the second leading cause of cancer-related death in men and the sixth most common cancer worldwide, with 830,180 deaths and an increasing incidence of over 900,000 new cases recorded in 2020 [1]. Liver cancer is prevalent specifically in Taiwan and Eastern Asia. Taiwan reported 10,988 new cases in 2020 [2], which equates to nearly 30 people receiving a diagnosis of liver cancer daily. Taiwanese government statistics suggest that among the 51,656 people who died of malignancy in 2021, 7,970 died of liver cancer [3]. Only lung cancer caused more deaths. From 1984 to 1998, liver cancer was the deadliest cancer in Taiwan, eventually surpassed by lung cancer in 1999 [4, 5].
Liver cancer is frequently diagnosed late in its course because of the absence of symptoms in patients with early-staged cancer. Only regular screening and follow-up in outpatient departments (OPDs) can enable the early diagnosis of liver cancer [6, 7]. If liver cancer is discovered during an emergency department (ED) visit, the symptoms or complications are typically severe [8]. Currently, Taiwan uses the Barcelona Clinic Liver Cancer (BCLC) classification as the standard staging system, which divides the disease into stages 0, A, B, C, and D [9]. The liver cancer stage is a factor affecting survival [10]. According to a cancer care report from a Taiwanese medical center, the 5-year relative survival rate of patients with liver cancer at stages 0 to D was 74%, 70.9%, 36.5%, 16.6%, and 11.2%, respectively [11]. In addition, the average cost of drugs for liver cancer treatment was NT$59,780 and the average medical cost per patient was NT$162,276 per person in 2018 [12]. When considering 5-year survival as a case of successful treatment, sustaining each patient costs an average of NT$2,273,554 [13].
Several studies have confirmed that hepatitis B and C are the main cause of liver cancer in Taiwan [14, 15]. A survey of individuals with hepatitis revealed that 85.9% of patients undertaking regular self-paid liver cancer screenings and follow-ups were in BCLC stages 0 and A at the time of diagnosis, whereas only 39% of the people who did not follow up regularly were in the early disease stages (0 or A) [16]. This demonstrates that regular screening and follow-up can effectively detect liver cancer earlier. However, the cancer screening and adult health examination services currently subsidized by the Taiwan government do not include liver cancer, and free hepatitis B and C screenings are only provided once in a lifetime for qualified persons [17].
Accordingly, studies have noted that regular OPD-based screenings can detect liver cancer at an earlier stage. However, no study has investigated the difference between the cancer stage and survival of patients with liver cancer who received an initial diagnosis in an OPD versus those who received such diagnosis in an ED in Taiwan to explore the effect of current adult health examination policy on the prevention of liver cancer.
Methods
Data sources
In this research, a retrospective cohort study design was adopted. The data for this study were obtained from the 2 million representative samples recorded in the 2000–2016 National Health Insurance Research Database (NHIRD), which was used to enroll study participants. We also linked this data with that of the Taiwan Cancer Registry from 2003 to 2015 and the Ministry of Health and Welfare’s Cause of Death File from 2003 to 2016. The Taiwan Cancer Registry contained information on cancer cases as well as relevant information such as patients’ cancer stage and time of cancer diagnosis. Cancer was diagnosed in accordance with the International Classification of Diseases (ICD) for Oncology, third edition (ICD-O-3), which identifies cancer categories according to the primary site, histology, behavioral code, and classification or differentiation. In determining the liver cancer stage according to the diagnostic results, the Taiwan Cancer Registry recorded data on the clinical, surgical, and pathological severity of cancers in accordance with the BCLC staging system.
Study population
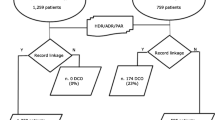
In this study, patients who received an initial diagnosis of liver cancer (ICD, 9th revision, Clinical Modification code 155, 10th revision of ICD and ICD-O-3 codes C22.0–C22.1) between 2003 and 2015 were selected as the study participants and were followed up until December 31, 2016. To evaluate the effect of utilization of the preventive health examination offered to Taiwanese adults over 40 years old, only patients aged over 40 years were enrolled in the study. The participants were retrospectively tracked from 2000 onwards to determine whether they received adult health examinations within 3 years of receiving an initial liver cancer diagnosis. The last visit date with relevant diagnosis and treatment for liver cancer before the cancer registration was acquired to determine the location of diagnosis (i.e. OPD or ED). Patients with the following conditions were excluded: liver cancer staging based on a system other than the BCLC, unknown liver cancer stage, incomplete details of the location of diagnosis, incomplete details of residential area (urbanization), incomplete details of education level, and incomplete medical institution data (Fig. 1).
Definition of relevant variables
The study variables were as follows: location of first-ever liver cancer diagnosis (OPD: 2,704 patients, ED: 177 patients); patient characteristics (e.g. sex, age, and marital status [unmarried, married, divorced, or widowed]); monthly salary (≤ NT$22,800, 22,801–28,800, or > 28,800); education level (elementary school or lower, junior high school, senior high school or higher); urbanization of residence areas (7 levels, with level 1 being the most urbanized) [18]; level of preventive health utilization (completed adult health exam during the 3 years before liver cancer diagnosis); level of health care organization (medical center, regional hospital, district hospital, or primary clinic); ownership status of the medical organization (public or non-public); and health status in terms of Charlson comorbidity index (CCI) score and hepatitis B and C diagnoses. CCI score was determined based on the patients’ primary and secondary diagnosis codes either twice in the OPD or once during hospitalization 1 year prior to the diagnosis of liver cancer and assigned a score of ≤ 2, 3–7, or ≥ 8 [19]; Hepatitis B or C infection were determined based on whether the patient data included diagnosis codes for hepatitis B or C twice in the OPD or once during hospitalization 1 year prior to the diagnosis of liver cancer.
Statistical methods
Descriptive data of the seven study variables, namely the location of diagnosis, personal characteristics, socioeconomic status, environmental factors, preventive health utilization, health status, and characteristics of the main hospital before diagnosis, are presented as numbers and percentages. The chi-squared test was used to assess whether the variables differed significantly between the OPD and ED patient groups. Multivariate logistic regression was performed to explore their correlation with the initial location of diagnosis and liver cancer stage (early or advanced). Log-rank test was performed to determine whether the survival of patients with liver cancer among patients differed significantly based on the location of diagnosis. Cox proportional hazard regression models were employed to estimate the adjusted hazard ratio (aHR) of death after controlling for confounders, including the location of diagnosis, personal characteristics, socioeconomic status, environmental factors, use of preventive health, health status, and characteristics of the primary care institutions. The follow-up duration was from the time of diagnosis of liver cancer to the time the patient withdrew from the NHI claims scheme, death, or December 31, 2016. The statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC, USA), and P values of < 0.05 were considered statistically significant. This study was approved by the research ethics committee of China Medical University and Hospital (Institutional Review Board No. CRREC-109-156).
Results
In this study, analyses of descriptive statistics were initially conducted to determine the basic characteristics of the two patient groups (with liver cancer diagnoses from the OPD or ED). A total of 2,881 patients were enrolled in this study, of which 2,704 (93.86%) and 177 (6.14%) received an initial liver cancer diagnosis in an OPD and ED, respectively (Table 1). In terms of staging, the majority of diagnoses received in an OPD were of stage A liver cancer (42.53%), whereas those received in an ED were primarily of stage C (36.72%). In terms of sex, male patients outnumbered their female counterparts in both the OPD and ED groups. Male patients accounted for 70.67% and 73.45% of the OPD and ED group members, respectively. In both groups, the majority of the patients were aged 55–64 years (30.88% and 32.20% in the OPD and ED groups, respectively). The chi-squared test results revealed that the following variables differed significantly (P < 0.05) between the OPD and ED groups: cancer stage, age at diagnosis, marital status, monthly salary, health examination in the 3 years prior to diagnosis, severity of comorbidities, hepatitis B infection, and the post-diagnosis medical institution level.
The multivariate logistic regression results indicated that patients who received an initial liver cancer diagnosis in an ED were more likely to be at an advanced disease stage (stage C: adjusted odds ratio [aOR] = 3.40, 95% confidence interval [CI] = 1.19–9.68; Stage D: aOR = 5.36, 95% CI = 1.68–17.14), divorced (aOR = 2.97, 95% CI = 1.18–7.50), have lower monthly salary, CCI score of ≥ 8 (aOR = 2.17, 95% CI = 1.02–4.63), not have hepatitis B (aOR = 1.61, 95% CI = 1.11–2.33), and in a non-public hospital (aOR = 1.62, 95% CI = 1.01–2.58). However, the use of health examination did not exert a significant effect on the likelihood of liver cancer diagnosis in an ED (adjusted odds ratio = 0.86, 95% CI = 0.61–1.21, P = 0.381; Table 1).
In this study, the impact of each variable on the stage of liver cancer was further analyzed. Table 2 indicates that compared with liver cancer cases initially diagnosed in OPDs, those initially diagnosed in EDs had higher proportions of stages B (28.81% vs. 24.89%), C (36.72% vs. 21.71%), and D (9.04% vs. 3.03%); by contrast, the proportions of stage 0 (2.26% vs. 7.84%) and A (23.16% vs. 42.53%) liver cancer cases diagnosed in an ED were lower than those of cases diagnosed in an OPD (P < 0.05).
Table 3 details the results of the multivariate logistic regression used to explore the factors related to the probability of advanced-stage liver cancer among the study participants, revealing significant differences based on the location of initial diagnosis, age at diagnosis, monthly salary, education level, use of preventive health examination, CCI score, hepatitis B and C infections, and the ownership of the main hospital attended before diagnosis (P < 0.05). The aOR of advanced-staged liver cancer diagnosis was 1.77 times higher in the ED group than in the OPD group (95% CI = 1.27–2.47, P < 0.001). Therefore, receiving an initial liver cancer diagnosis in an ED entailed a higher probability of advanced-stage cancer than receiving a diagnosis in an OPD. In addition, patients who have received adult health examinations within the 3 years before diagnosis had a 0.76 times lower risk of advanced stage liver cancer (95% CI = 0.62–0.92, P = 0.005) compared with those who did not receive such an examination.
Table 4 presents the results of the Cox proportional hazard model used to explore the risk of liver cancer-specific death in the patients and related factors. Significant differences were observed based on the location of diagnosis, cancer stage, sex, monthly salary, education level, degree of urbanization of the residential area, use of preventive health services, and severity of comorbidities (P < 0.05). After adjustment for the study variables, the risk of death in the ED group patients was greater than that in the OPD group patients (aHR = 1.38, 95% CI = 1.14–1.68, P = 0.001). An association between initial ED diagnosis and poorer survival remains even after adjustment for the cancer stage at the time of diagnosis. Furthermore, Fig. 2 depicts the survival curves of patients who received an initial liver cancer diagnosis in an OPD versus those who received such a diagnosis in an ED after controlling for related variables and the survival curves of liver cancer patients at each cancer stage (Fig. 2). The patients with a higher monthly salary had a lower risk of death, with patients who received a monthly salary of NT$22,801–28,800 and > 28,800 exhibiting aHRs of 0.01 (95% CI = 0.01–0.03) and 0.03 (95% CI = 0.01–0.05), respectively. The patients with a higher education level also had a lower risk of death, with those having an education level of junior high school and senior high school (above) registering aHRs of 0.82 (95% CI = 0.70–0.97) and 0.78 (95% CI = 0.67–0.91), respectively. As for the residential area, the risk of death among the patients residing in places with level 4–7 urbanization was higher than that of those in level 1 areas (aHR = 1.27–1.72). The risk of death in patients who completed a health examination within 3 years prior to initial cancer diagnosis was 0.64 times (95% CI = 0.57–0.72) that of those who had not undergone such an examination. In terms of the severity of comorbidities, patients with liver cancer and a CCI score of ≥ 8 had a higher risk of death (aHR = 1.45, 95% CI = 1.06–1.98, P < 0.001) than those with a CCI score < 8.
Discussion
The results of this study revealed that most of the liver cancer cases initially diagnosed in OPDs were stage A (42.53%), whereas most of those diagnosed in EDs were stage C (36.72%). Having stage C or D cancer, low monthly salary, CCI score ≥ 8, being divorced, not having hepatitis B, and attending a non-public hospital as the primary care institution before diagnosis were risk factors for initial ED diagnosis (P < 0.05, Table 1). Studies have confirmed that patients who received ED-based liver cancer diagnoses are more likely to be men, have advanced-staged cancer, and exhibit a higher incidence of large tumors and metastatic diseases that also yield symptoms such as decompensated liver cirrhosis, ascites, varicose bleeding, abdominal pain, and weight loss [8]. One study has also shown that in OPDs, the progression from liver disease to liver cancer can be monitored every year, and the detection rate is 1.6% [20]. Another study revealed that half of the local lesions detected by outpatient ultrasound were liver cancer cases and most of the patients received diagnoses of BCLC stage 0 or A cancer [21]. Therefore, cancer cases diagnosed in an OPD are mostly detected at an early stage, and the results of our study also indicate that the proportion of early-staged cancer diagnoses (stages 0, A, and B) was higher in OPDs than in EDs (75.26% vs. 54.23%). The aOR of develo** advanced-stage cancer was 1.77 times higher in the ED group than in the OPD group (Table 3), which accords with the results of [8].
Although an ideal liver cancer diagnosis policy would involve screening patients with risk factors in outpatient clinics, because most high-risk patients have limited access to medical services, many US citizens (32%) receive an initial liver cancer diagnosis in an ED [8], where the proportion of uninsured individuals is higher. Our research revealed that Taiwan’s proportion of initial liver cancer diagnoses in EDs (6.14%) is lower than that of the United States. This may be due to Taiwan’s implementation of the NHI scheme. In the United Kingdom, which has a universal healthcare system, cancers of different organs are initially diagnosed in EDs in 13.9–29% of cases, but no case of liver cancer was initially detected in an ED in two British studies [22, 23]. This may be because of the low prevalence of liver cancer in the United Kingdom (approximately a third of that in Taiwan or Eastern Asia) [24] or because most patients with liver cancer have been screened out in the OPD. Therefore, compared with the United Kingdom, Taiwan still has room for improvement in outpatient screening and the prevention of liver cancer. In South Korea, the National Cancer Screening Program provides free liver cancer screening services twice a year for the low-income insured (bottom 50%) [25]. Although no studies yet have shown that the program has the effect of reducing liver cancers diagnosed in the ED, it is still a good policy that the Taiwan government can emulate.
The present study demonstrates that advanced stage liver cancer (aOR = 0.76, 95% CI = 0.62–0.92, P = 0.005; Table 3) and death (OR = 0.64, 95% CI = 0.57–0.72, P < 0.001; Table 4) were less likely among those who received adult health examinations. However, the use of health examination did not exert a significant effect on the likelihood of liver cancer diagnosis in an ED (aOR = 0.86, 95% CI = 0.61–1.21, P = 0.381; Table 1). At present, the adult preventive health care service subsidized by the Taiwan government provides a free health exam every 3 years for people aged over 40 and under 65 years. Moreover, indigenous people aged over 55 years, people with poliomyelitis sequelae aged over 35 years, and people aged over 65 years are entitled to one free health exam every year. The examination items include basic questionnaire surveys (inquiring into disease and family history), physical examinations (general physical examinations, blood pressure, and body mass index), laboratory examinations (urinalysis and basic blood biochemical examinations), and health consultations (regarding quitting alcohol and betel nut consumption and maintaining a healthy diet and weight) [26]. People born after 1966 and aged over 45 years or indigenous people aged over 40 years can receive a free hepatitis B and C screening once in their lives [27]. Abdominal ultrasonography and α-fetoprotein analysis, which are recommended for liver cancer detection [6], are not provided in these adult preventive health examinations or hepatitis B and C screening services. Regarding cancer screening, the government currently provides screening services for four cancers, namely cervical, breast, oral, and colorectal cancers [17]. Liver cancer screening is not included in the cancer screening service in Taiwan.
Our results also revealed that most liver cancer patients with hepatitis B and C receive cancer diagnoses at a non-advanced stage, which was similar to the findings of a previous Japanese study [28]. The reason may be that people with hepatitis B and C have regular follow-up treatments, and liver cancer is therefore largely detected and controlled early. In a Swiss study, all of the participating patients with liver cancer received a diagnosis at an early stage through ultrasound examination in hepatology outpatient clinics [21]. For the years spanning 2012 to 2015, the numbers of people in Taiwan who were screened for hepatitis B and C were 38,000, 57,000, 76,000, and 89,000, respectively; the positive rates of hepatitis B and C screening were approximately 16.0–16.3%, and 3.8–4.7%, respectively, and these rates have continued to increase [29]. In a sample survey of 4,928 people aged 25–69 years, 69.1% had been tested for hepatitis B and C, and 12% received diagnoses of hepatitis B or C after being screened. After becoming aware they had hepatitis B or C, 69% of people sought medical treatment. As of 2017, a total of 476,341 people had received hepatitis C screening services in Taiwan, and the average hepatitis C positive screening rate was 4.3% [30]. Therefore, a considerable proportion of people have still not received hepatitis B and C screening or have not received further treatment after receiving hepatitis B or C diagnoses.
Studies have found that married patients are less likely to have advanced tumors. Social support from the spouses was associated with lower cortisol levels and higher natural-killer cells which may result in decreasing tumor progression. The spouses may also encourage patients to receive definitive versus expectant management [31, 32]. Our study results also indicated that the probability of receiving an advanced-staged cancer diagnosis is lower in married patients than in unmarried people, although the difference is non-significant (aOR = 0.69, 95% CI = 0.47–1.01, P = 0.055). Our study results also revealed that the higher the monthly salary, the lower the probability of receiving a late-stage cancer diagnosis (Table 3), which is consistent with the results of previous studies [33].
According to the results of this study, the risk of liver cancer-specific death was 1.38 times higher in the ED group than in the OPD group (95% CI = 1.14–1.68, P < 0.001). ED diagnosis of liver cancer is associated with poorer survival even after adjustment of other variables (Table 4). The survival curves of the OPD and ED groups (Fig. 2) also indicate that the 1-, 3-, and 5-year survival rates were lower in the ED group than in the OPD group. A South Korean study reported that liver cancer is one of the most common cancers in the ED and the in-hospital mortality was 19.7% [34]. Another South Korean study reported that 24% of patients with liver cancer that ruptured in the ED died within 30 days and 50.4% died within 90 days [35], which also demonstrated a similar poor outcome in the ED.
This study’s results suggest that compared with patients with stage 0 liver cancer, the risk of death for patients with stage B, C, and D liver cancer were 1.47, 2.90, and 4.32 times higher, respectively. This indicates that the more advanced the liver cancer is, the higher the risk of death (Table 4), a finding consistent with the results of previous studies [36, 37]. The results of our study reveal that women with liver cancer have a significantly lower risk of death than men (aHR = 0.87, 95% CI = 0.75–1.00, P = 0.046; Table 4). This finding is consistent with the results of previous studies. Female sex has a protective effect on liver cancer progression [38]. One study revealed that the prognosis of women with liver cancer is superior to that of men, and women tend to be diagnosed at an older age and an earlier stage (stage 0 or A) [39]. In our study, the risk of death among patients in level 4 to level 7 (least urbanized) residential areas was higher than that among patients in highly urbanized cities (level 1), with aHRs of 1.27–1.72. Studies have shown that the risk of liver cancer mortality among rural residents is greater than that of urban residents [33, 40]. This distinction may relate to medical care and resource disparities between urban and rural areas. The relative survival rate of urban residents is reportedly over twice that of rural residents [40], which is consistent with our findings. Our study of comorbidity revealed that the higher the severity of the comorbidity, the higher the risk of death. A previous study reported that compared with patients with a CCI score of ≤ 5, the risk of death in patients with scores of 6 and ≥ 7 were 1.5 and 2.5 times greater, respectively [41]. Our study results suggest that the patients with CCI scores of ≥ 8 had a higher risk of death than those with lower scores (aHR = 1.45, 95% CI = 1.06–1.98, P = 0.020).
Limitations
To our knowledge, this is the first cohort study to compare ED and OPD-diagnosed liver cancers in Taiwan, which has comprehensive population coverage by NHI (coverage rate of 99.7%) [42]. A similar existing study was conducted in the US with only 55% population having government insurance. The strengths of our study are the population-based design, long study period, and large sample size. However, several limitations must be noted. Because the NHIRD was used for analysis in this study, only the reimbursement data could be presented, and it was impossible to know whether patients undertook self-paid health examinations. In addition, miss-coding of the liver cancer ICD codes in the ED may also have consequences for the underestimation of liver cancer patients in the ED. Finally, patients may have visited an ED for temporary treatment but then been referred to receive further confirmatory examinations in the OPD, which may have resulted in an underestimation of the number of liver cancer cases diagnosed in EDs and an overestimation of those diagnosed in OPDs.
Conclusion
This study suggests that liver cancer cases initially diagnosed in EDs tend to be more advanced in stage than those diagnosed in OPDs (45.76% vs. 24.74%). Although patients who have received prior adult health examination are less likely to receive advanced stage cancer diagnoses (aOR = 0.76, 95% CI = 0.62–0.92, P = 0.005; Table 3) or die from cancer (aOR = 0.62, 95% CI = 0.56–0.69, P < 0.001; Table 4), the current Taiwanese health examination service (without liver cancer screening) is insufficient to ensure that cancer diagnoses are received in OPDs rather than EDs (P = 0.381). The patients who receive initial liver cancer diagnosis in EDs are at a higher risk of advanced stage cancer (aOR = 1.77, 95% CI = 1.27–2.47, P < 0.001) and death (aHR = 1.38, 95% CI = 1.14–1.68, P = 0.001) even after adjustment of other related variables. The government may consider further implementing liver cancer screening for high-risk and low-socioeconomic people for early detection and treatment.
Data Availability
In this study, three databases were used, namely the NHIRD, the Taiwan Cancer Registry, and the Cause of Death File. Data are available from the Ministry of Health and Welfare, Taiwan. Due to legal restrictions imposed by the Taiwanese government related to the Personal Information Protection Act, we cannot make these databases publicly available. Researchers can apply for access to use them in their studies. Requests for data can be sent as a formal proposal to the Science Centre of the Ministry of Health and Welfare (https://www.mohw.gov.tw/mp-2.html). Removing raw data from the Science Centre is not permitted. Only the analytic outputs in the form of tables or figures can be printed out.
Abbreviations
- ED:
-
Emergency Department
- OPD:
-
Outpatient departments
- CI:
-
Confidence interval
- NT$:
-
New Taiwan Dollar
- BCLC:
-
Barcelona Clinic Liver Cancer
- NHIRD:
-
National Health Insurance Research Database
- ICD:
-
International Classification of Diseases
- ICD-O-3:
-
International Classification of Diseases for Oncology, third edition
- CCI:
-
Charlson comorbidity index
- aHR:
-
Adjusted hazard ratio
- aOR:
-
Adjusted odds ratio
References
Liver cancer fact sheet. [https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf]
Cancer Registration Report. (2020) [https://www.hpa.gov.tw/File/Attach/16434/File_20339.pdf]
Summary Table of Causes of Death in 2021. In. Taiwan: Ministry of Health and Welfare; 2022.
Chen CH, Chen DS. [Hepatocellular carcinoma: 30 years’ experience in Taiwan]. J Formos Med Assoc. 1992;91(Suppl 3):187–202.
Cancer Registry Annual Report of. 1998 [https://www.hpa.gov.tw/File/Attach/6505/File_6170.pdf]
Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–22.
Kansagara D, Papak J, Pasha AS, O’Neil M, Freeman M, Relevo R, Quiñones A, Motu’apuaka M, Jou JH. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261–9.
Kronenfeld JP, Ryon EL, Goldberg D, Lee RM, Yopp A, Wang A, Lee AY, Luu S, Hsu C, Silberfein E, et al. Disparities in presentation at Time of Hepatocellular Carcinoma diagnosis: a United States Safety-Net Collaborative Study. Ann Surg Oncol. 2021;28(4):1929–36.
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38.
Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, Chen CH, Changchien CS, Hsu HC, Hu TH, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46(4):744–51.
Report on Cancer Care in the Chung Shang Medical University Hospital. in 2015 [http://web.csh.org.tw/web/cancer/wp-content/uploads/2017/09/2015ver.pdf]
Cancer cost ranking of. 2018 [https://www.nhi.gov.tw/DL.aspx?sitessn=292&u=LzAwMS9Gc1VwbDYyMzEvMjkyL3JlbGZpbGUvMC8yODU5NC8xMDflubTlkITpoZ7nmYznl4flgaXkv53liY0xMOWkp%2BmGq%2BeZguaUr%2BWHuue1seioiC0wOTEzLnBkZg%3D%3D&n=MTA35bm05ZCE6aGe55mM55eH5YGl5L%2Bd5YmNMTDlpKfphqvnmYLmlK%2Flh7rntbHoqIgtMDkxMy5wZGY%3D&ico%20=.pdf]
Huang CF, Hung HC. Longitudinal analysis for Hepatocellular Carcinoma’s Medical Resource utilization. J Healthc Manag. 2008;9(4):243–54.
Sun CA, Farzadegan H, You SL, Lu SN, Wu MH, Wolfe L, Hardy W, Huang GT, Yang PM, Lee H, et al. Mutual confounding and interactive effects between hepatitis C and hepatitis B viral infections in hepatocellular carcinogenesis: a population-based case-control study in Taiwan. Cancer Epidemiol Biomarkers Prev. 1996;5(3):173–8.
Lu SN, Su WW, Yang SS, Chang TT, Cheng KS, Wu JC, Lin HH, Wu SS, Lee CM, Changchien CS, et al. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int J Cancer. 2006;119(8):1946–52.
Mo LR, Li SF. The impact of regular surveillance of hepatocellular carcinoma on overall survival of patients with hepatitis B or C. Show-Chwan Med J. 2015;14(12):12–9.
Introduction to Cancer Screening. [https://www.hpa.gov.tw/Pages/List.aspx?nodeid=211]
Liu C-Y, Hung Y-T, Chuang Y-L, Chen Y-J, Weng W-S, Liu J-S, Liang K. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4(1):1–22.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Sherman M. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28(5):783–93.
Frey RS, Boldanova T, Heim M. Ultrasound surveillance for hepatocellular carcinoma: real-life performance in a hepatology outpatient clinic. Swiss Med Wkly. 2015;145:w14200.
Abel GA, Mendonca SC, McPhail S, Zhou Y, Elliss-Brookes L, Lyratzopoulos G. Emergency diagnosis of cancer and previous general practice consultations: insights from linked patient survey data. Br J Gen Pract. 2017;67(659):e377–87.
Tsang C, Bottle A, Majeed A, Aylin P. Cancer diagnosed by emergency admission in England: an observational study using the general practice research database. BMC Health Serv Res. 2013;13:308.
Estimated age-standardized incidence rates (World) in 2020, liver, both sexes, all ages [https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D]
Kang HT. Current status of the National Health Screening Programs in South Korea. Korean J Fam Med. 2022;43(3):168–73.
Adult Preventive Health Care [https://www.hpa.gov.tw/Pages/List.aspx?nodeid=189]
Hepatitis B. and C screening [https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=1115&pid=13580]
Akada K, Koyama N, Taniguchi S, Miura Y, Aoshima K. Database analysis of patients with hepatocellular carcinoma and treatment flow in early and advanced stages. Pharmacol Res Perspect. 2019;7(4):e00486.
Viral hepatitis prevention. and treatment plan [https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/10114/File_11608.pdf]
Current status of. hepatitis C screening, treatment, and prevention in Taiwan [https://www.mohw.gov.tw/dl-52701-d0692584-ed8e-493a-b65b-fcf8d25fae38.html]
Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76.
Zhang W, Wang X, Huang R, ** K, Zhangyuan G, Yu W, Yin Y, Wang H, Xu Z, Sun B. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep. 2017;7:41695.
Wong RJ, Kim D, Ahmed A, Singal AK. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer. 2020;127(1):45–55.
Kim YJ, Seo DW, Kim WY. Types of cancer and outcomes in patients with cancer requiring admission from the emergency department: a nationwide, population-based study, 2016–2017. Cancer. 2021;127(14):2553–61.
Lee S-H, Kim J-S, Yu GN, Kim Y-J, Ryoo SM, Sohn CH, Kim WY, Ahn S. Factors prognostic of ruptured hepatocellular carcinoma presenting to the emergency department. J Korean Soc Emerg Med. 2019;30(6):521–8.
Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS ONE. 2014;9(3):e90929.
Xu LB, Wang J, Liu C, Pang HW, Chen YJ, Ou QJ, Chen JS. Staging systems for predicting survival of patients with hepatocellular carcinoma after surgery. World J Gastroenterol. 2010;16(41):5257–62.
Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, El-Khoueiry A. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and end results analysis. Cancer. 2014;120(23):3707–16.
Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;52(4):701–9.
Zheng R, Zuo T, Zeng H, Zhang S, Chen W. [Mortality and survival analysis of liver cancer in China]. Zhonghua Zhong Liu Za Zhi. 2015;37(9):697–702.
Shinkawa H, Tanaka S, Takemura S, Amano R, Kimura K, Nishioka T, Miyazaki T, Kubo S. Predictive value of the age-adjusted Charlson Comorbidity Index for Outcomes after hepatic resection of Hepatocellular Carcinoma. World J Surg. 2020;44(11):3901–14.
Wu T-Y, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. Lond J Prim care. 2010;3(2):115–9.
Acknowledgements
We are grateful to the Health Data Science Centre, China Medical University Hospital, for providing administrative, technical, and financial support.
Funding
This work was supported by China Medical University Hospital, Taiwan (grant numbers DMR-108-226).
Author information
Authors and Affiliations
Contributions
TYH, JJY, SYY, HYT, WYC, PTK, and WCT developed concepts and methodology; TYH, PTK, and WCT obtained data sources. TYH, JJY, SYY, HYT, WYC, PTK, and WCT contributed data analysis and interpretation; JJY, SYY, HYT, and WYC conducted data curation and projection administration; TYH and WCT obtained financial sources; TYH, JJY, SYY, HYT, and WCT wrote the draft of the manuscript; TYH, PTK, and WCT revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been performed in accordance with the Declaration of Helsinki. Since this study was a retrospective data analysis and did not involve human participants, the informed consent was waived, and the study was approved by the research ethics committee of China Medical University and Hospital (Institutional Review Board No. CRREC-109-156).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hsu, TY., Ye, JJ., Ye, SY. et al. Disparities in the first-ever diagnosed liver cancers between the emergency department and outpatient department in Taiwan: a population-based study. BMC Public Health 23, 283 (2023). https://doi.org/10.1186/s12889-023-15218-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15218-5