Abstract
Background
IgA vasculitis nephritis (IgAVN) and IgA nephropathy (IgAN) share several clinical and pathological characteristics, though distinctions also exist. Their interrelation, however, remains undefined. This study investigates the clinicopathological divergences and prognostic disparities in pediatric patients with IgAVN and IgAN.
Methods
Our study encompasses 809 pediatric patients with IgAVN and 236 with IgAN, all of whom underwent kidney biopsy. We utilized the Semiquantitative Classification (SQC) scoring system to juxtapose the pathologies of the two conditions, and performed a COX regression analysis to examine factors influencing their prognoses.
Results
Both patient groups demonstrated a predominance of males. A seasonality was observed, with a higher incidence of IgAN in the summer, and IgAVN in the fall (P < 0.0001). Patients with IgAN exhibited more severe tubulointerstitial injury, higher chronicity index, and total biopsy scores compared to those with IgAVN (P < 0.0001). Mesangial deposition intensity of complement C3, and the rate of pure IgA deposition, were found to be greater in patients with IgAVN compared to those with IgAN (P < 0.0001). The intensity of IgA deposition was also significantly higher in IgAVN patients (P = 0.003). IgAVN demonstrated a superior prognosis, with a higher rate of kidney remission (P < 0.0001). COX regression analysis indicated that interstitial fibrosis, as identified in the SQC pathology system, was associated with the prognosis of both conditions. Furthermore, the findings suggest that IgA deposition levels (IgA + + and IgA + + +) could potentially influence the prognosis of IgAVN.
Conclusions
Compared to IgAVN, IgAN manifests more severely with regard to renal impairment, interstitial damage, and prognosis. The disparities in immune complex deposition levels and locations within the kidneys support the hypothesis of IgAVN and IgAN as distinct diseases. Interstitial fibrosis may serve as a key pathological indicator within the SQC system associated with kidney prognosis in children with IgAVN and IgAN. The degree of IgA deposition could also be linked with the prognosis of IgAVN.
Similar content being viewed by others
Introduction
IgA vasculitis (IgAV), a prominent type of vasculitis among pediatric populations, is marked by its capacity to impact small blood vessels in multiple organs, including the skin, and is distinguished by the presence of immunofluorescent IgA deposits. This condition is specifically termed IgA vasculitis nephritis (IgAVN) when it affects the kidneys [Treatment Steroids combined with RARS were used in children with combined proteinuria at a starting steroid dose of 1.5–2 mg/kg/d, and steroids combined with immunosuppressants were used in children with proteinuria levels greater than 50 mg/kg/d; the commonly used immunosuppressant is cyclophosphamide. We recommend methylprednisolone pulse in cases of crescents with more than 25% of glomeruli. Grade A (complete remission): normal kidney function, without proteinuria or hematuria; Grade B (partial remission): persistent proteinuria (< 1.0 g/d), and/or hematuria (≥ 3 red blood cells/high power field) without kidney insufficiency; Grade C (no remission): persistent proteinuria (≥ 1.0 g/d), and/or hematuria with moderate kidney failure (< 30% decrease in the eGFR from the baseline); Grade D (kidney failure): ≥ 30% decline in the eGFR from the baseline, End-stage kidney disease (ESRD) or death. IgAVN and IgAN pediatric patients' kidney remission rates were compared using a plot of the Kaplan–Meier survival analysis of survival curves. Data analysis was executed using SPSS for windows version 26 (IBM Corporation, Armonk, NY). Normally distributed data were expressed as the mean ± standard deviation (SD), and nonparametric data were expressed as the median and interquartile (IQR) range. The count data were expressed as percentages, and Chi-squared Test or Fisher's exact probability test was used for group comparison. Nonparametric variables were compared with the Kruskal–Wallis test. Kidney survival rates were analyzed by the Kaplan–Meier method and compared by log-rank test. The Cox proportional hazards regression model was conducted on univariable and multivariable analyses of SQC classification in the IgAVN and IgAN children. Univariable Cox regression analysis was performed for each pathological variable and deposition intensity of IgA and C3. Multivariable Cox regression analysis was performed to appraise the influence of SQC system and deposition intensity of IgA and C3 on the kidney outcome. Hazard ratio (HR) with 95% confidence interval (CI) for each variable was estimated. All probabilities were two-tailed, and P < 0.05 was considered statistically significant.Kidney prognosis
Statistical analysis
Results
Comparison of clinical data
Clinical baseline characteristics are provided in Table 1. IgAN and IgAVN showed higher prevalence during the spring and winter seasons, while IgAVN was more common in the autumn (P < 0.0001). As for clinical symptoms, IgAN presented a higher incidence of gross hematuria, whereas IgAVN was more likely to manifest as isolated microscopic hematuria (P < 0.0001). Conversely, IgAVN was associated with a higher incidence of hypertension (P = 0.005).
Comparison of kidney pathological changes
IgAN was found to inflict more severe kidney tubulointerstitial injury compared to IgAVN (P < 0.0001). According to the SQC, cellular crescents were more prevalent in IgAVN glomerular lesions (P = 0.001). Additionally, statistically significant differences were observed between IgAVN and IgAN concerning the pathological scores for complete tubular atrophy (P < 0.0001), interstitial fibrosis (P = 0.002), interstitial or periglomerular inflammation (P = 0.002), capillary arteriosclerosis or arterial inflammation (P < 0.0001), and diffuse mesangial proliferation (P < 0.0001). IgAN demonstrated a higher chronicity index and total biopsy score compared to IgAVN (P < 0.0001). Further details can be found in Table 2.
Comparison of immunopathology data
IgAVN frequently exhibits IgA + + + deposits, whereas IgA + + deposition is more common in IgAN, and the difference is statistically significant (P = 0.003). While both IgAVN and IgAN demonstrate C3 + deposition dominance, the proportion of C3 + is considerably higher in IgAN (P < 0.0001). Moreover, IgA deposits are typically localized to the mesangium in IgAVN, whereas IgAN displays a higher propensity for IgA deposition within both the mesangium and the segmental loop (P < 0.0001). Further details can be found in Table 3.
Comparison of treatments and kidney prognosis of IgAVN and IgAN
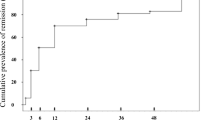
The therapeutic measures and kidney prognosis are elaborated in Table 4. No significant distinction exists in treatment between IgAVN and IgAN. Both conditions receive more Renin-Angiotensin System Blocker (RASB) therapy, with a relatively low rate of immunosuppressant usage, implying a preponderance of patients with mild disease in our study cohort. The 1-, 3-, and 5-year kidney remission rates were markedly lower in the IgAN group compared to the IgAVN group (70.8% vs. 71.1%, 40.1% vs. 52.0%, and 25.1% vs. 40.0%; Log-rank test, P = 0.017) as depicted in Fig. 2. To investigate the impact of the Semiquantitative Scoring (SQC) system's pathological indicators and the intensity of immune complex IgA and C3 deposition on kidney remission rates of IgAVN and IgAN, Cox proportional hazard models were established, as shown in Table 5.
The Univariate Cox analysis model revealed associations between interstitial fibrosis, inflammation OR periglomerular inflammation, diffuse mesangial proliferation, intensity of immune complex IgA deposition, and adverse outcomes of IgAVN. Additionally, adverse outcomes of IgAN correlated with the presence of fibrous crescents, complete tubular atrophy, interstitial fibrosis, and inflammation OR periglomerular inflammation. In the multivariate Cox analysis, interstitial fibrosis and IgA + + and IgA + + + appeared to be linked to poor kidney outcomes for IgAVN. For IgAN, only interstitial fibrosis was associated with poor kidney prognosis. Regarding IgAVN, when the intensity of IgA deposition varied, we compared kidney remission rates (Fig. 3). Expectedly, children with IgAVN exhibited significantly lower kidney remission rates when IgA deposition intensity reached + + + , indicating a pronounced impact of IgA + + + on prognosis.
Discussion
Our study findings highlighted that both IgAVN and IgAN have a higher prevalence in males, with a relatively larger proportion in IgAN. The SQC chronicity index and total biopsy score were higher for IgAN than for IgAVN, which also exhibited more severe tubular interstitial injury. The deposition intensity of C3, and the rate of IgA pure deposition in the mesangium, were higher in IgAVN than in IgAN, with the intensity of IgA deposition also greater in IgAVN. Notably, IgAVN had a more favorable prognosis and a higher kidney remission rate. According to Cox regression, interstitial fibrosis in the SQC pathology system correlated with the prognosis of both IgAVN and IgAN. Further, IgA + + and IgA + + + might impact the prognosis of IgAVN. Our study also demonstrated a significantly higher incidence of IgAVN during autumn compared to IgAN [21].
Additionally, we noted some significant distinctions; IgAN patients were more prone to gross hematuria than those with IgAVN. Fengmei Wang et al. [22] reported that patients with IgAVN and IgAN have a higher incidence of experiencing left kidney vein compression (nutcracker phenomenon) than those with other kidney diseases. Children with IgAN also reported higher rates of hematuria than those with IgAVN. This correlation warrants further investigation.
Koskela et al. [23] performed serial kidney biopsies on the cohort and assessed the prognostic value of the SQC system for IgAVN in children. They found higher activity scores at diagnostic biopsy, increasing activity and chronicity scores at follow-up biopsy, and that the activity scores declined while chronicity scores increased from the diagnostic biopsy to the follow-up biopsy in most patients, irrespective of proteinuria. The Cox regression results indicated that activity scores at the diagnostic biopsy independently predicted chronicity scores at the follow-up biopsy.
In another study, the authors applied the SQC system to score kidney biopsies in 80 patients with IgAVN and compared the prognostic implications of the International Study of Kidney Disease in Children (ISKDC) and SQC classifications. They found that patients with active kidney disease had higher SQC activity and chronicity scores. The area under the curve was not significantly different between the two at Receiver Operating Characteristic (ROC) curve analysis, demonstrating the effective applicability of the SQC system to patients with IgAVN [24]. A recent multicenter study also suggested that the SQC and Oxford classifications predicted poor kidney prognosis in IgAVN more accurately than the ISKDC classification [25].
In our study, we utilized the SQC system for the first time to assess the severity of kidney lesions in children with IgAVN and IgAN. Compared to the Oxford classification, the SQC system pays more attention to interstitial glomerular lesions. We discovered that pathological scores for complete tubular atrophy, interstitial fibrosis, interstitial inflammation or periglomerular inflammation, and capillary arteriosclerosis or arterial inflammation were higher in IgAN than in IgAVN. Moreover, the chronicity index and total biopsy scores were higher in IgAN, suggesting that kidney pathological changes in IgAN are more severe and demonstrate a chronic pathological course [26]. Our investigation revealed significantly more severe tubulointerstitial lesions in IgAN than in IgAVN, corroborating our findings.
In a retrospective study conducted in Japan, it was discerned that IgAN demonstrated a higher incidence of mesangial proliferation compared to IgAVN [27]. This observation found corroboration in a similar study executed in China [2]. Consistent with these previous findings, our research also showed a more pronounced degree of diffuse mesangial proliferation in IgAN than in IgAVN. The prognostic predictive value of M lesions under the Oxford classification was impacted by the use of immunosuppression, as discovered by Yu et al. [15]. In a similar vein, Shima et al. [28] found that lesion M lost its predictive power in patients undergoing immunosuppressive therapy. In the context of our study, we identified no significant disparity in the utilization rate of immunosuppressants between IgAVN and IgAN. The lack of correlation between mesangial proliferation and kidney prognosis in COX regression univariate and multivariate analyses might be attributed to the analogous mesangial proliferation scores in both groups.
This research found no divergence in the distribution of complex immune types deposited in IgAVN and IgAN. Furthermore, we observed no noticeable differences in the rates of IgG and IgM deposition in the kidneys of IgAVN and IgAN patients, which stood at 40.2% and 38.6%, 73.7% and 74.2% (data not shown), respectively. Mao et al.'s study investigating the clinicopathological relationship between IgAVN and IgAN in children unveiled a striking discrepancy in the blood biochemical parameters of IgAVN and IgAN when the types of immune complex deposition varied [29]. This may be connected to the pathogenesis variability of both conditions. An analysis of the relationship between immune complexes and clinical manifestations remains to be conducted in this paper, and future research is required for more comprehensive understanding.
Our findings indicate significant IgA deposition in the glomerular mesangial zone in both IgAVN and IgAN. Results derived from immunofluorescence deposition intensity revealed that IgA + + + predominated in IgAVN, whereas IgA + + was more prevalent in IgAN. This suggests a higher intensity of IgA deposition in IgAVN, which could be relevant given the primarily acute alterations in the kidney pathophysiology of IgAVN. When a membrane attack complex (MAC) was deposited in the glomerular mesangial zone of IgAVN and IgAN patients' kidneys, alongside IgA and C3 deposits, patients exhibited more severe kidney impairment when MAC was highly expressed, as evidenced by a Canadian study [30]. Consequently, MAC may serve as an independent indicator of kidney injury severity in IgAVN and IgAN patients. However, the relationship between the degree of IgA and C3 deposition and MAC remains to be established. Future investigations are necessary to elucidate their connection to the severity of kidney injury and prognosis. IgAVN and IgAN both demonstrated a predilection for IgA deposition in the glomerular mesangium. In contrast, IgAN exhibited a higher incidence of IgA deposition in both the glomerular mesangium and the segment loop. The increased frequency of IgA deposition in the segment loop might be linked to the more conspicuous capillary alterations associated with IgAN. Furthermore, our study found no discernible disparities in the rates of C1q, C4, and FRA deposition between IgAVN and IgAN.
Our Kaplan–Meier survival analysis unveiled a notably superior prognosis for IgAVN, marked by significantly higher kidney remission rates at 1, 3, and 5 years in comparison to IgAN. As per a specific study, only 30–50% of patients diagnosed with IgAN reach the phase of clinical remission, contrasting sharply with nearly 98% of patients afflicted with IgAVN [31]. These findings align with our research. Through our Cox regression analysis, we ascertained that interstitial fibrosis of the semiquantitative score (SQC) and the strength of IgA deposition may be intertwined with the prognosis of IgAVN, while interstitial fibrosis of SQC was associated with the prognosis of IgAN. Given the comprehensive comparison, interstitial fibrosis emerged as a key determinant influencing kidney outcomes [32,33,34,35].
Furthermore, our research identified that cellular crescents were more prevalent in glomerular lesions of IgAVN, implying an acute trajectory of kidney lesions in IgAVN. Huang et al.'s study posited that the severity of kidney pathological presentations in IgAVN escalates with the proportion of crescents [36]. In contrast, our study did not establish a correlation between crescents and the prognosis of IgAVN. The connection between the percentage of crescents and kidney prognosis remains to be elucidated. Wu et al.'s study noted that 6.46% of children with IgAN progressed to kidney failure with cumulative kidney survival rates of 95.3%, 90.3%, and 84% at 5, 10, and 15 years, respectively [37]. Another study highlighted that 20% of children diagnosed with IgAN reached end-stage kidney disease (ESRD) 20 years post-diagnosis, underlining a gradual decline in prognosis in children with IgAN [38]. Given our study's relatively brief follow-up period, none of the children reached the stage of kidney insufficiency.
The strengths of our study include the large sample size, persuasive clinical comparisons, and the novel application of SQC for scoring and accurately assessing kidney pathology in IgAVN and IgAN. Despite these strengths, our study also has limitations: it is a single-center retrospective study, potentially carrying geographical differences and selection bias. Moreover, the data on clinical, laboratory, and treatment parameters are relatively undeveloped, the impact of treatment on pathological tissues remains unclear, the follow-up period is relatively brief, and our prognostic model only incorporates SQC pathological indicators. Future multicenter prospective studies are required to address these limitations.
Conclusions
Despite certain clinical and pathological similarities, IgAN exhibits more severe kidney impairment, conspicuous interstitial damage, and a poorer prognosis than IgAVN. Significant differences are observed in the intensity and location of immune complex deposition in the kidney. These differences do not support the notion of these two diseases being identical; rather, they suggest two distinct pathologies. The SQC pathological indicator associated with kidney prognosis in children with IgAVN and IgAN could be interstitial fibrosis. The intensity of IgA deposition may be linked to the prognosis of IgAVN.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IgAVN:
-
IgA vasculitis nephritis
- IgAN:
-
IgA nephropathy
- CI:
-
Confidence intervals
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- SQC:
-
Semiquantitative classification
- PAS:
-
Periodic Acid-Schiff
- PAM:
-
Periodic acid-silver methenamine
- RASB:
-
Renin-angiotensin system blockade
- MAC:
-
Membrane attack complex
- ESRD:
-
End-stage kidney disease
- ISKDC:
-
International Study of Kidney Disease in Children
References
Zhang X, **e X, Shi S, Liu L, Lv J, Zhang H. Plasma galactose-deficient immunoglobulin A1 and loss of kidney function in patients with immunoglobulin A vasculitis nephritis. Nephrol Dial Transplant. 2020;35(12):2117–23.
Li X, Tang M, Yao X, Zhang N, Fan J, Zhou N, et al. A clinicopathological comparison between IgA nephropathy and Henoch-Schönlein purpura nephritis in children: use of the Oxford classification. Clin Exp Nephrol. 2019;23(12):1382–90.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–545.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–556.
Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–21.
Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80(3):310–7.
Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult chinese patients. Am J Kidney Dis. 2012;60(5):812–20.
Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Tsuchimoto A, Noguchi H, et al. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8(12):2082–90.
Asrar I, Hussain M, Afzal A, Hassan U, Ishtiaq S. Blind Spot in the Radar of MEST-C Score: Type and Severity of Tubulointerstitial Nephritis in IgA Nephropathy. Int J Nephrol. 2023;2023:1060526.
Rankin AJ, Kipgen D, Geddes CC, Fox JG, Milne G, Mackinnon B, et al. Assessment of active tubulointerstitial nephritis in non-scarred renal cortex improves prediction of renal outcomes in patients with IgA nephropathy. Clin Kidney J. 2018;12(3):348–54.
Koskela M, Ylinen E, Ukonmaanaho EM, Autio-Harmainen H, Heikkilä P, Lohi J, et al. The ISKDC classification and a new semiquantitative classification for predicting outcomes of Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2017;32(7):1201–9.
Huang X, Ma L, Ren P, Wang H, Chen L, Han H, et al. Updated Oxford classification and the international study of kidney disease in children classification: application in predicting outcome of Henoch-Schönlein purpura nephritis. Diagn Pathol. 2019;14(1):40.
Suzuki H, Yasutake J, Makita Y, Tanbo Y, Yamasaki K, Sofue T, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93(3):700–5.
Yun D, Kim DK, Oh KH, Joo KW, Moon KC, Kim YS, et al. MEST-C pathological score and long-term outcomes of child and adult patients with Henoch-Schönlein purpura nephritis. BMC Nephrol. 2020;21(1):33.
Yu B, Shi S, Hou W, Liu L, Lv J, Wang S, et al. Evaluation of the Oxford classification in immunoglobulin A vasculitis with nephritis: a cohort study and meta-analysis. Clin Kidney J. 2020;14(2):516–25.
Wang M, Wang R, He X, Zhang P, Kuang Q, Yao J, et al. Using MEST-C Scores and the International Study of Kidney Disease in Children Classification to Predict Outcomes of Henoch-Schönlein Purpura Nephritis in Children. Front Pediatr. 2021;9: 658845.
Jimenez A, Chen A, Lin JJ, South AM. Does MEST-C score predict outcomes in pediatric Henoch-Schönlein purpura nephritis? Pediatr Nephrol. 2019;34(12):2583–9.
Luo X, Tan J, Wan D, Chen J, Hu Y. Predictability of the Oxford classification of IgA nephropathy in Henoch-Schonlein purpura nephritis. Int Urol Nephrol. 2022;54(1):99–109.
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806.
Bohle A, Müller GA, Wehrmann M, Mackensen-Haen S, **ao JC. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl. 1996;54:S2–9.
Calvo-Río V, Loricera J, Martín L, Ortiz-Sanjuán F, Alvarez L, González-Vela MC, et al. Henoch-Schönlein purpura nephritis and IgA nephropathy: a comparative clinical study. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S45–51.
Wang F, Zhu H, Bao S, Qi H, Xu L, Liu X, et al. Associations of left renal vein entrapment with IgA nephropathy and Henoch-Schönlein purpura nephritis. Ren Fail. 2022;44(1):1519–27.
Koskela M, Ylinen E, Autio-Harmainen H, Tokola H, Heikkilä P, Lohi J, et al. Prediction of renal outcome in Henoch-Schönlein nephritis based on biopsy findings. Pediatr Nephrol. 2020;35(4):659–68.
Yel S, Dursun I, Pinarbaşi AS, Günay N, Özdemir S, Şahin N, et al. Patient Outcomes of Henoch-Schönlein Purpura Nephritis According to the New Semiquantitative Classification. Fetal Pediatr Pathol. 2020;39(5):381–9.
Kifer N, Bulimbasic S, Sestan M, Held M, Kifer D, Srsen S, et al. Correction to: Semiquantitative classification (SQC) and Oxford classifications predict poor renal outcome better than The International Study of Kidney Disease in Children (ISKDC) and Haas in patients with IgAV nephritis: a multicenter study. J Nephrol. 2023;36(2):593.
Sugiyama M, Wada Y, Kanazawa N, Tachibana S, Suzuki T, Matsumoto K, et al. A cross-sectional analysis of clinicopathologic similarities and differences between Henoch-Schönlein purpura nephritis and IgA nephropathy. PLoS ONE. 2020;15(4): e0232194.
Komatsu H, Fujimoto S, Yoshikawa N, Kitamura H, Sugiyama H, Yokoyama H. Clinical manifestations of Henoch-Schönlein purpura nephritis and IgA nephropathy: comparative analysis of data from the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol. 2016;20(4):552–60.
Shima Y, Nakanishi K, Kamei K, Togawa H, Nozu K, Tanaka R, et al. Disappearance of glomerular IgA deposits in childhood IgA nephropathy showing diffuse mesangial proliferation after 2 years of combination/prednisolone therapy. Nephrol Dial Transplant. 2011;26(1):163–9.
Mao S, Xuan X, Sha Y, Zhao S, Zhu C, Zhang A, et al. Clinico-pathological association of Henoch-Schoenlein purpura nephritis and IgA nephropathy in children. Int J Clin Exp Pathol. 2015;8(3):2334–42.
Dumont C, Mérouani A, Ducruet T, Benoit G, Clermont MJ, Lapeyraque AL, et al. Clinical relevance of membrane attack complex deposition in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol. 2020;35(5):843–50.
Hastings MC, Rizk DV, Kiryluk K, Nelson R, Zahr RS, Novak J, et al. IgA vasculitis with nephritis: update of pathogenesis with clinical implications. Pediatr Nephrol. 2022;37(4):719–33.
Kim CH, Lim BJ, Bae YS, Kwon YE, Kim YL, Nam KH, et al. Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch-Schönlein purpura nephritis in adults. Mod Pathol. 2014;27(7):972–82.
Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6(9):2175–84.
Hao Y, Zhao Y, Huang R, Fu P. Analysis of the relationship between Oxford classification, IgM deposition and multiple indexes and the adverse prognosis of patients with primary IgA nephropathy and related risk factors. Exp Ther Med. 2019;17(2):1234–9.
Zhong ZX, Tan JX, Tang Y, Tan L, Pei GQ, Qin W. Crescent lesions are not a predictive factor in adult-onset Henoch-Schönlein purpura nephritis. Clin Exp Med. 2019;19(4):449–56.
Huang X, Wu J, Wu XM, Hao YX, Zeng CH, Liu ZH, et al. Significance of histological crescent formation in patients with IgA vasculitis (Henoch-Schönlein purpura)-related nephritis: a cohort in the adult Chinese population. BMC Nephrol. 2018;19(1):334.
Wu H, Fang X, **a Z, Gao C, Peng Y, Li X, et al. Long-term renal survival and undetected risk factors of IgA nephropathy in Chinese children-a retrospective 1243 cases analysis from single centre experience. J Nephrol. 2020;33(6):1263–73.
Coppo R. Pediatric IgA Nephropathy in Europe. Kidney Dis (Basel). 2019;5(3):182–8.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of Jiangxi Province (No. 20192BAB205023) and the Postgraduate Innovation of Jiangxi Province (No. YC2022-s201).
Author information
Authors and Affiliations
Contributions
LY and PX designed the research, conducted analysis and interpretation of data, and revised the article critically with FR; DY, WY, and YT contributed to data acquisition and assisted in data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by Jiangxi Children's Hospital Ethics Committee (Reference number JXSETYY-YXKY-2019098). Jiangxi Children's Hospital Ethics Committee waived the requirement for informed consent in consideration of the retrospective nature of the study and anonymous data analyses.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lv, Y., Fu, R., Peng, XJ. et al. Comparative study on clinicopathological features and prognosis of IgA vasculitis nephritis and IgA nephropathy in children. BMC Pediatr 23, 423 (2023). https://doi.org/10.1186/s12887-023-04243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04243-3