Abstract
Background
Local recurrence after surgery and radiochemotherapy seriously affects the prognosis of locally advanced rectal cancer (LARC) patients. Studies on molecular markers related to the radiochemotherapy sensitivity of cancers have been widely carried out, which might provide valued information for clinicians to carry out individual treatment.
Aim
To find potential biomarkers of tumors for predicting postoperative recurrence.
Methods
In this study, LARC patients undergoing surgery and concurrent radiochemotherapy were enrolled. We focused on clinicopathological factors and PTEN, SIRT1, p-4E-BP1, and pS6 protein expression assessed by immunohistochemistry in 73 rectal cancer patients with local recurrence and 76 patients without local recurrence.
Results
The expression of PTEN was higher, while the expression of p-4E-BP1 was lower in patients without local recurrence than in patients with local recurrence. Moreover, TNM stage, lymphatic vessel invasion (LVI), PTEN and p-4E-BP1 might be independent risk factors for local recurrence after LARC surgery combined with concurrent radiochemotherapy.
Conclusions
This study suggests that PTEN and p-4E-BP1 might be potential biomarkers for prognostic prediction and therapeutic targets for LARC.
Similar content being viewed by others
Background
In recent years, the incidence of colorectal cancer (CRC) in China has been increasing continuously, and it has become the fifth most fatal malignant tumor [1]. As a number of studies have confirmed that concurrent radiochemotherapy can reduce local recurrence after rectal cancer surgery, adjuvant/neoadjuvant radiochemotherapy has become a standard treatment for stage II/III rectal cancer in China [2,3,4]. However, the rate of local recurrence in these patients remains at approximately 6.6–13.6% [5], which seriously affects the prognosis.
Previous studies showed that some clinicopathological features were associated with postoperative recurrence of rectal cancer [6, 7]. Recently, studies on molecular markers related to radiochemotherapy sensitivity of cancers have been widely carried out [8,9,10,11], which might play a positive role in ameliorating the implementation of the ‘watch and wait’ strategy after neoadjuvant radiochemotherapy or promoting accurate implementation of adjuvant radiochemotherapy for locally advanced rectal cancer (LARC) patients.
Mammalian target of rapamycin (mTOR), a serine/threonine kinase, has regulatory effects on cell proliferation, DNA damage repair, cell apoptosis and autophagy [12, 13], which is regulated by the PI3K-Akt axis and negatively regulated by PTEN and SIRT1 [14,15,16]. Moreover, 4E-BP1 and S6RP are the main downstream target proteins of mTOR. Previously, we carried out laboratory studies on the function and regulatory factors of mTOR. It was found that ionizing radiation could induce sustained activation of mTOR and promote the levels of pS6, p-4E-BP1 and p-Akt473 in rectal adenocarcinoma cells (not published). However, inhibition of mTOR could prolong the G0/G1 phase of the cell cycle and reduce DNA damage repair efficiency via attenuation of homologous recombination, which suggests that mTOR might play important roles in the radiosensitivity of rectal adenocarcinoma cells.
In this study, LARC patients who underwent surgery and preoperative/postoperative radiochemotherapy at Tian** Union Medical Center, Nankai University were enrolled, and a retrospective clinical study was carried out. We investigated clinicopathological features and expression of mTOR-related proteins in patients with or without local recurrence and explored the risk factors for local recurrence of LARC after surgery.
Methods
Patients
The clinical data of 1599 rectal cancer patients who received radiotherapy with concurrent 5-fluorouracil (5-FU) based chemotherapy and total mesorectal excision (TME) surgery were collected from January 2012 through May 2020, from Tian** Union Medical Center, Nankai University. Clinical staging was classified by endosonography, computed tomography (CT) scan, and magnetic resonance imaging (MRI). The inclusion criteria were as follows; (1) pathologically diagnosed rectal adenocarcinoma; (2) cT3, cT4 or N + according to American Joint Committee on Cancer (AJCC) criteria (7th edition); (3) no evidence of distant metastasis; and (4) Karnofsky performance status over 70. To perform the study, 109 recurrent patients were selected, and another 109 patients were randomly selected from 1490 non recurrent ones. Sixty-nine patients with incomplete pathologic data or follow up data were excluded, reducing the total number of patients analyzed to 149, which were divided into a local recurrence group (LR, n = 73) and a nonrecurrence group (NR, n = 76).
Treatment
All patients underwent radiotherapy with conventional fractionation of 1.8 Gy up to a total dose of 45 Gy in 25 fractions. For patients undergoing preoperative radiotherapy, an additional 5.4 Gy in 3 fractions was delivered as a boost to the gross tumor volume (GTV). The clinical target volume (CTV) was defined as the GTV and regional lymphatics including the mesorectal, presacral, internal iliac, and distant common iliac lymph nodes. To cover the CTV properly, the borders of the pelvic fields were as follows: (1) superior: the sacral promontory at the L5-S1 junction level, (2) inferior: 3 to 5 cm distal to the GTV, (3) lateral: 1.5 cm lateral to the widest bony margin of the true pelvic walls, (4) anterior: posterior border of the symphysis pubis, and (5) posterior: 1.5 cm behind the anterior bony sacral margin. The concurrent chemotherapy regimens were as follows: (1) CAPEOX, two cycles of L-OHP (130 mg/m2 day1) and capecitabine (825 mg/m2 twice daily day1-14 ) given at the first, and fourth weeks of radiotherapy; (2) CAPE, oral capecitabine (825 mg/m2 twice daily day1-5) given at every week of radiotherapy; (3) mFOLFOX6, L-OHP (85 mg/m2 day1), calcium levofolinate (200 mg/m2 day1) and 5-Fu (400 mg/m2 bolus, 2400 mg/m2 civ 46 h) given at the first, third and fifth weeks of radiotherapy; and (4) Tegafur (20 mg/kg day1-3) given at the first, and fourth weeks of radiotherapy. Patients receiving neoadjuvant therapy underwent planned TME surgery 4–6 weeks after the end of radiotherapy, and patients receiving adjuvant therapy underwent TME surgery 4–8 weeks before radiotherapy.
Follow up
The patients were seen every 3 months during the first 2 years after surgery and every 6 months thereafter. Follow-up evaluations included physical examination, complete blood count, biochemical profile, abdominopelvic CT (or MRI), chest radiography, and proctoscopy (every 1 year).
Tissue microarray (TMA) construction
Tissue samples were fixed in buffered 4% formalin, paraffin embedded, and used for TMA construction. Hematoxylin-eosin–stained histologic sections from resection specimens were used to mark representative tumor areas. A 1.0-mm tissue core was punched out from the tumor area of each specimen and transferred to a TMA recipient block using a TM-1 tissue arrayer (Bilcool Inc, Bei**g, China). Three TMAs contained 149 tumor spots consisting of 73 rectal cancers of the local recurrence group and 76 rectal cancers of the nonrecurrence group.
Immunohistochemical staining
The 4-µm–thick sections from TMA were deparaffinized and rehydrated using xylene and ethanol, and immersed in a 3% hydrogen peroxide solution for 10 min to block endogenous peroxidase. The sections were then boiled for 30 min in 10 mM/L citrate buffer solution (pH 6.0) for antigen retrieval. Slides were incubated with 5% skim milk for 20 min and then with PTEN antibody (1:1000, #9559, Cell Signaling Technology, Danvers, MA, USA), SIRT1 antibody (1:2000, ab110304, Abcam Biotechnology, Cambridge, MA, UK), p-4E-BP1 antibody (1:1000, #2855, Cell Signaling Technology, Danvers, MA, US) and pS6 antibody (1:1000, #5364, Cell Signaling Technology, Danvers, MA, USA) at 4 °C for 16 h, and visualized using the SP-9000 Polymer Detection System (Golden Bridge International Corp, Wharton, TX, USA) following the manufacturer’s instructions. Subsequently, the slides were incubated with 3, 3′-diaminobenzidine tetrahydrochloride-H2O2 solution for visualization, and counterstained with hematoxylin. All slices were observed under an Olympus DS-Ri2 microscope (Olympus, Tokyo, Japan), and PTEN, SIRT1, p-4E-BP1, and pS6 staining was defined as yellow or brown particles in either the nucleus or the cytoplasm. Immunostained slides were evaluated by two experienced pathologists who were blinded to all clinical parameters. The staining results were evaluated using a semiquantitative method: the percentage of stained cells × the staining intensity. This percentage was scored as 0 for < 5%, 1 for 5–25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76–100%, and the staining intensity was then scored as 0 for no staining, 1 for light yellow, 2 for yellow brown, and 3 for brown. A score less than 4 is defined as negative expression, and a score greater than or equal to 4 is defined as positive expression [17].
Statistical analysis
The Chi-square test or Fisher’s exact test was used for categorical variables. Logistic regression analysis was applied to examine the factors associated with local recurrence. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 26.0 (IBM Inc., Chicago, IL, USA).
Results
Patient characteristics
We studied 149 rectal cancer patients, comprising 73 LR cases (50 males, 23 females) and 76 NR cases (50 males, 26 females). The median age of these patients at diagnosis was 61.2 years in the LR group and 59.1 years in the NR group. There was no significant difference between the LR and NR group with the distance of tumor from the anal verge. Histologically, 78 (52.3%) tumors were classified as well or moderately differentiated carcinoma, 66 (44.3%) tumors were classified as poorly differentiated, and 5 (3.4%) were uncertain. Ninety-nine (66.4%) tumors were smaller than 5 cm, and 50 (33.6%) tumors had a minimum diameter of 5 cm or more. N stage was N0 for 36 (24.2%) tumors, N1 for 67 (45.0%) tumors and N2 for 46 (30.9%). Fifty-one (34.2%) cases were classified as stage II and 98 (65.8%) cases were classified as stage III according to the TNM staging system. Seventeen (11.4%) patients had lymphatic vessel invasion (LVI) and 132 (88.6%) patients did not. All patients underwent TME with a curative aim, in which 83 (55.7%) patients received Dixon surgery, 43 (28.9%) patients received Miles surgery, and 23 (15.4%) patients received Hartmann surgery. Seventy-three (49.0%) patients underwent neoadjuvant radiochemotherapy, and 76 (51.0%) patients underwent adjuvant radiochemotherapy. However, there were 8 (5.4%) patients whose circumferential resection margin was involved and 2 (1.3%) patients whose distal margin was involved. The concurrent chemotherapy regimens during radiotherapy were CAPE (53, 35.6%), CAPEOX (30, 20.1%), mFOLFOX6 (64, 43.0%), or Tegafur (2, 1.3%). As shown in Tables 1 and 2, and Supplementary Table 1, LNM, TNM stage and LVI might be correlated with local recurrence, while other characteristics were similar between LR and NR patients.
PTEN and p-4E-BP1 might be associated with postoperative recurrence
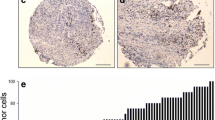
A total of 149 patient samples were subjected to immunohistochemical analysis in this study, and the results are shown in Fig. 1; Table 3. The PTEN protein, a negative regulator of mTOR, was mainly localized in the cytoplasm. The total positive rate of PTEN protein expression in all cases was 63.1% (94/149), and the positive rate in the LR group was 53.4% (39/73), which was lower than that in the NR group (72.4%, 55/76). The difference was statistically significant (P < 0.05). SIRT1 protein, mainly localized in the nucleus, can inhibit mTOR activity [16]. The total positive rate of SIRT1 protein expression in all patients was 83.2% (124/149), and there was no significant difference in SIRT1 expression between the LR group and the NR group (84.9% vs. 81.6%, P > 0.05). The p-4E-BP1 protein was mainly localized in the cytoplasm, and its expression level was positively correlated with mTOR activity. The total positive rate of p-4E-BP1 protein expression in all cases was 64.4% (96/149), of which 74.0% (54/73) vs. 55.3% (42/76) were in the LR group and NR group, respectively, and the difference was statistically significant (P < 0.05). PS6, another marker of mTOR activation protein, was mainly localized in the cytoplasm. The positive rate of pS6 protein expression in all patients was 79.2% (118/149), and there was no significant difference in pS6 expression between the LR group and the NR group (73.7% vs. 84.9%, P > 0.05).
We included meaningful clinical, pathological and protein expression results in the logistic multivariate regression analysis. As shown in Table 4, the results showed that TNM stage, LVI, PTEN negativity and p-4E-BP1 positivity were all independent risk factors for local recurrence after TME surgery combined with concurrent radiochemotherapy (P < 0.05).
Discussion
In this study, we focused on SIRT1-related protein (PTEN, SIRT1, p-4E-BP1, and pS6) expression as assessed by immunohistochemistry in 73 rectal cancer patients with local recurrence and 76 patients without local recurrence after TME surgery and concurrent radiochemotherapy. The expression of PTEN was higher while the expression of p-4E-BP1 was lower in patients without local recurrence than in patients with local recurrence, suggesting that mTOR activation might be implicated in the local recurrence of rectal cancer.
Before the era of preoperative radiochemotherapy became the standard treatment, several prognostic factors for predicting recurrence were reported [18, 19], such as tumor size, pathological T and N stages, histologic grade, vascular invasion, and carcinoembryonic antigen (CEA) level. In recent years, a part of LARC patients with neoadjuvant radiochemotherapy obtained ideal tumor regression, while it is difficult to obtain comprehensive pathological information, such as vascular invasion, tissue differentiation, and pTNM stage [3, 5].
It is widely accepted that currently only clinical-pathological information will not be robust enough to have prognostic and predictive utility. Recently, molecular biomarkers, involved in cell proliferation, apoptosis, DNA damage repair, cell cycle, angiogenesis, and epithelial to mesenchymal transition have been widely investigated [20, 21]. These include tumor tissue biomarkers of either biopsy or surgical specimens, and blood-based biomarkers as well as tumor related nucleic acids and circulating tumor cells (CTCs). As yet, not a single marker is adequate to have clinical utility. Screening biomarkers of tumors by using microarrays would likely have higher yield and low cost, and it is expected that a combination of markers would prove most useful [22, 23]. Biopsy, blood, and CTCs could be obtained for checking biomarkers at different stages of patients’ diagnosis and treatment, which would be expected to provide valuable information for clinicians to fulfill individual treatment.
The PI3K/mTOR signaling pathway, modulating tumor initiation and development in many types of cancers, is a potential candidate to predict the response to radiochemotherapy [24,25,26]. The main regulator of this system is PTEN, which can stop signaling by removing 3’-phosphate groups. Therefore, the PI3K/mTOR pathway contributes to the phosphorylation of 4E-BP1, which results in the formation of the cap-dependent mRNA translation initiation complex [27], and the regulation of protein synthesis, cell growth and proliferation. In recent years, many scholars were concerned about the potential linkage between loss of PTEN and chemotherapy resistance to cetuximab in advanced colorectal cancer [28,29,30,31,32,33,34]. A few studies have discussed the relationship between PTEN and radiation sensitivity. Philipp’s study and Dellas’s study suggested PTEN expression do not predict efficacy of cetuximab-based radiochemotherapy in LARC [35, 36]. In Oya’ s work [37], basal mRNA expression level of PTEN has been investigated, and it was suggested PTEN could be a potential predictive elements for radiochemotherapy. Compared with PTEN, fewer works about 4E-BP1 were reported. Chen’s work [12] manifested that p-4E-BP1 might be a biomarker to predict chemotherapy outcome of patients with colorectal cancer, but not radiotherapy outcome. Our study showed that PTEN and p-4E-BP1 were expressed in LARC, and multivariate analysis in 169 patients demonstrated that the levels of PTEN and p-4E-BP1 were significantly associated with local recurrence after surgery combined with radiochemotherapy. Our results are in agreement with previous studies in esophageal squamous cell carcinoma, renal cell carcinoma, hilar cholangiocarcinoma, and lung cancer [38,39,40,41].
Conclusion
The results of this study suggest that PTEN and p-4E-BP1 might be potential tumor markers for prognostic prediction and a therapeutic target for LARC. Although no predictive biomarkers for response to LARC are sufficiently robust to have clinical utility, the integration of diverse types of biomarkers, including clinicopathological and imaging features will allow the development of a sensitive molecular biomarker panel and enhance efforts for personalized care in rectal cancer patients.
Data availability
The data sets that support the conclusions of this article are included within the article.
Abbreviations
- LARC:
-
locally advanced rectal cancer
- LVI:
-
lymphatic vessel invasion
- CRC:
-
colorectal cancer
- mTOR:
-
mammalian target of rapamycin
- TME:
-
total mesorectal excision
- CT:
-
computed tomography
- MRI:
-
magnetic resonance imaging
- AJCC:
-
American Joint Committee on Cancer
- LR:
-
local recurrence
- NR:
-
nonrecurrence
- GTV:
-
gross tumor volume
- CTV:
-
clinical target volume
- TMA:
-
tissue microarray
- CTCs:
-
circulating tumor cells
- CEA:
-
carcinoembryonic antigen
References
**a C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90.
Smith FM, Winter D. Pathologic Complete Response of Primary Tumor Following Preoperative Chemoradiotherapy for locally advanced rectal Cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09 – 01). Ann Surg. 2017;265(4):e27–8.
Nussbaum N, Altomare I. The neoadjuvant treatment of rectal cancer: a review. Curr Oncol Rep. 2015;17(3):434.
Lee JW, Lee JH, Kim JG, Oh ST, Chung HJ, Lee MA, Chun HG, Jeong SM, Yoon SC, Jang HS. Comparison between preoperative and postoperative concurrent chemoradiotherapy for rectal cancer: an institutional analysis. Radiation Oncol J. 2013;31(3):155–61.
van Oostendorp SE, Smits LJH, Vroom Y, Detering R, Heymans MW, Moons LMG, Tanis PJ, de Graaf EJR, Cunningham C, Denost Q, et al. Local recurrence after local excision of early rectal cancer: a meta-analysis of completion TME, adjuvant (chemo)radiation, or no additional treatment. Br J Surg. 2020;107(13):1719–30.
Kim HG, Kim HS, Yang SY, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Early recurrence after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: characteristics and risk factors. Asian J Surg. 2021;44(1):298–302.
Nishimuta M, Hamada K, Sumida Y, Araki M, Wakata K, Kugiyama T, Shibuya A, Hashimoto S, Ozeki K, Morino S, et al. Long-term prognosis after surgery for locally recurrent rectal Cancer: a retrospective study. Asian Pac J cancer Prevention: APJCP. 2021;22(5):1531–5.
Romain B, Meyer N, Brigand C, Chenard MP, Schneider A, Guenot D. Molecular markers for recurrence and sensitivity to preoperative chemoradiotherapy in locally advanced rectal tumours. Dig Surg. 2014;31(4–5):347–53.
Tie J, Cohen JD, Wang Y, Li L, Christie M, Simons K, Elsaleh H, Kosmider S, Wong R, Yip D, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019;68(4):663–71.
Shen L, van Soest J, Wang J, Yu J, Hu W, Gong YU, Valentini V, **ao Y, Dekker A, Zhang Z. Validation of a rectal cancer outcome prediction model with a cohort of Chinese patients. Oncotarget. 2015;6(35):38327–35.
Bhangu A, Wood G, Mirnezami A, Darzi A, Tekkis P, Goldin R. Epithelial mesenchymal transition in colorectal cancer: seminal role in promoting disease progression and resistance to neoadjuvant therapy. Surg Oncol. 2012;21(4):316–23.
Chen Y, Wang J, Fan H, **e J, Xu L, Zhou B. Phosphorylated 4E-BP1 is associated with tumor progression and adverse prognosis in colorectal cancer. Neoplasma. 2017;64(5):787–94.
Li S, Kong Y, Si L, Chi Z, Cui C, Sheng X, Guo J. Phosphorylation of mTOR and S6RP predicts the efficacy of everolimus in patients with metastatic renal cell carcinoma. BMC Cancer. 2014;14:376.
Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960–76.
Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36(16):2191–201.
Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE. 2010;5(2):e9199.
Wang LL, Hao S, Zhang S, Guo LJ, Hu CY, Zhang G, Gao B, Zhao JJ, Jiang Y, Tian WG, et al. PTEN/PI3K/AKT protein expression is related to clinicopathological features and prognosis in breast cancer with axillary lymph node metastases. Hum Pathol. 2017;61:49–57.
Kim JY, Chung SM, Choi BO, Lee IK, An CH, Won JM, Ryu MR. Prognostic significance of the lymph node ratio regarding recurrence and survival in rectal cancer patients treated with postoperative chemoradiotherapy. Gut Liver. 2012;6(2):203–9.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Lim SH, Chua W, Henderson C, Ng W, Shin JS, Chantrill L, Asghari R, Lee CS, Spring KJ, de Souza P. Predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Critical reviews in oncology/hematology 2015, 96(1):67–80.
Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A. Predictive and prognostic molecular biomarkers for response to Neoadjuvant Chemoradiation in rectal Cancer. Int J Mol Sci 2017, 18(3).
Conde-Muino R, Cuadros M, Zambudio N, Segura-Jimenez I, Cano C, Palma P. Predictive Biomarkers to Chemoradiation in Locally Advanced Rectal Cancer. BioMed research international 2015, 2015:921435.
Bohn BA, Mina S, Krohn A, Simon R, Kluth M, Harasimowicz S, Quaas A, Bockhorn M, Izbicki JR, Sauter G, et al. Altered PTEN function caused by deletion or gene disruption is associated with poor prognosis in rectal but not in colon cancer. Hum Pathol. 2013;44(8):1524–33.
Subtil FSB, Grobner C, Recknagel N, Parplys AC, Kohl S, Arenz A, Eberle F, Dikomey E, Engenhart-Cabillic R, Schotz U. Dual PI3K/mTOR Inhibitor NVP-BEZ235 Leads to a Synergistic Enhancement of Cisplatin and Radiation in Both HPV-Negative and -Positive HNSCC Cell Lines. Cancers 2022, 14(13).
Shiratori H, Kawai K, Okada M, Nozawa H, Hata K, Tanaka T, Nishikawa T, Shuno Y, Sasaki K, Kaneko M, et al. Metastatic role of mammalian target of rapamycin signaling activation by chemoradiotherapy in advanced rectal cancer. Cancer Sci. 2020;111(4):1291–302.
Wanigasooriya K, Barros-Silva JD, Tee L, El-Asrag ME, Stodolna A, Pickles OJ, Stockton J, Bryer C, Hoare R, Whalley CM, et al. Patient derived Organoids Confirm that PI3K/AKT signalling is an escape pathway for Radioresistance and a target for therapy in rectal Cancer. Front Oncol. 2022;12:920444.
Wang J, Ye Q, She QB. New insights into 4E-BP1-regulated translation in cancer progression and metastasis. Cancer cell Microenvironment 2014, 1(5).
Negri FV, Bozzetti C, Lagrasta CA, Crafa P, Bonasoni MP, Camisa R, Pedrazzi G, Ardizzoni A. PTEN status in advanced colorectal cancer treated with cetuximab. Br J Cancer. 2010;102(1):162–4.
Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Annals Oncology: Official J Eur Soc Med Oncol. 2009;20(1):84–90.
Razis E, Briasoulis E, Vrettou E, Skarlos DV, Papamichael D, Kostopoulos I, Samantas E, Xanthakis I, Bobos M, Galanidi E, et al. Potential value of PTEN in predicting cetuximab response in colorectal cancer: an exploratory study. BMC Cancer. 2008;8:234.
Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, Camponovo A, Etienne LL, Cavalli F, Mazzucchelli L. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97(8):1139–45.
Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2009;27(35):5924–30.
Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2009;27(16):2622–9.
Li FH, Shen L, Li ZH, Luo HY, Qiu MZ, Zhang HZ, Li YH, Xu RH. Impact of KRAS mutation and PTEN expression on cetuximab-treated colorectal cancer. World J Gastroenterol. 2010;16(46):5881–8.
Erben P, Strobel P, Horisberger K, Popa J, Bohn B, Hanfstein B, Kahler G, Kienle P, Post S, Wenz F, et al. KRAS and BRAF mutations and PTEN expression do not predict efficacy of cetuximab-based chemoradiotherapy in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):1032–8.
Dellas KRF, KapplerM. DA, B MHF, R DVC. EGFR, PTEN, and survivin expression in pre- and posttherapeutic specimens does not predict a clinical benefit from preoperative chemoradiation with cetuximab in patients (pts) with locally advanced rectal cancer (LARC). J Clin Oncol. 2010;28(15):3653.
Orun O, Ozden S, Kilinc O, Mega Tiber P, Yonar P, Ozgen Z, Ozyurt H. The role of the PTEN/mTOR axis in clinical response of rectal cancer patients. Mol Biol Rep. 2022;49(9):8461–72.
Fang Z, Lu L, Tian Z, Luo K. Overexpression of phosphorylated 4E-binding protein 1 predicts lymph node metastasis and poor prognosis of Chinese patients with hilar cholangiocarcinoma. Med Oncol. 2014;31(5):940.
Roh MS, Lee JH, Kang KW, Nam HY, Jung SB, Kim K, Lee EH, Park MI, Kim MS, Lee HW. Phosphorylated 4E-binding protein 1 expression is associated with poor prognosis in small-cell lung cancer. Virchows Archiv: Int J Pathol. 2015;467(6):667–73.
Lee HW, Lee EH, Lee JH, Kim JE, Kim SH, Kim TG, Hwang SW, Kang KW. Prognostic significance of phosphorylated 4E-binding protein 1 in non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(4):3955–62.
Chao YK, Chuang WY, Yeh CJ, Chang YS, Wu YC, Kuo SY, Hsieh MJ, Hsueh C. High phosphorylated 4E-binding protein 1 expression after chemoradiotherapy is a predictor for locoregional recurrence and worse survival in esophageal squamous cell carcinoma patients. J Surg Oncol. 2012;105(3):288–92.
Acknowledgements
We are grateful for the excellent work of Qiong Xu, Lin Qiu, Feng Cao and **nyue Fang.
Funding
This study was supported by the National Natural Science Foundation of China [81972847], Tian** Key Medical Discipline (Specialty) Construction Project [TJYXZDXK-053B], and Foundation of Tian** Union Medical Center (2022ZLXK07).
Author information
Authors and Affiliations
Contributions
HZ, HQW, XPZ, and HW participated in the design of the study, data analyses, and manuscript preparation. WJS, and HRQ collected patients’ clinical and pathological data. XFL, and HY participated in the TMA construction and immunohistochemical staining. SWZ, and HY participated in the histological analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involving human participants was performed in compliance with the Declaration of Helsinki and was approved by an ethics committee of Tian** Union Medical Center. Informed consent to participate in this survey was obtained from all participants.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Li, X., Sun, W. et al. PTEN and P-4E-BP1 might be associated with postoperative recurrence of rectal cancer patients undergoing concurrent radiochemotherapy. BMC Cancer 24, 582 (2024). https://doi.org/10.1186/s12885-024-12339-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12339-x