Abstract
Background
Immunotherapy based on the application of immune checkpoint inhibitors (ICIs) is one of the standard treatments for advanced non-small cell lung cancer (NSCLC). Non-alcoholic fatty liver Disease (NAFLD) has demonstrated predictive value for response to immunotherapy in non-lung cancer types. Our study investigated the effect of NAFLD on the efficacy of real-life use of ICIs for patients with stage III / IV NSCLC.
Methods
The clinical and imaging data of patients with stage III / IV NSCLC who were first admitted to Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from March 2020 to July 2022 were retrospectively collected to ensure that they underwent at least one CT scan before treatment. A total of 479 patients were divided into the NAFLD group (Liver/Spleen density ratio ≤ 1) and the non-NAFLD group (Liver/Spleen density ratio > 1) by measuring the baseline liver and spleen CT value. The overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and disease control rate (DCR) of the patients were obtained.
Results
A total of 118 patients with NAFLD and 361 patients without NAFLD were included in the study. Patients with NAFLD tended to have higher BMI and higher total bilirubin compared to patients without NAFLD. The median duration of follow-up in the study was 22 m (IQR, 17–29 m). Both of 2 groups had a higher DCR (94% vs. 92%, p = 0.199) and ORR (38.1% vs. 44.9%, p = 0.452) respectively. There was no difference in efficacy between the two groups. In univariate analysis, NAFLD had no significant effect on PFS (p = 0.785) and OS (p = 0.851). Surprisingly, the presence of hypertension was observed to be associated with a higher OS (HR 1.471 95%CI 1.018–2.127, p = 0.040). Besides, based on multivariate analysis, lactic dehydrogenase was associated with PFS (HR 1.001 95%CI 1.000,1.002, p = 0.037) and OS (HR 1.002, 95%CI 1.001–1.003, p < 0.001).
Conclusions
Among patients with NSCLC, NAFLD did not result in changes in survival or disease progression after immune checkpoint inhibitor therapy.
Similar content being viewed by others
Introduction
Over the past decade, the introduction of immunotherapy has had a profound impact on the outlook for individuals diagnosed with non-small cell lung cancer (NSCLC) [1]. Immune checkpoint inhibitors (ICIs) such as PD-1 and PD-L1 have emerged as key therapeutic agents by obstructing the inhibitory pathways that normally regulate immune responses. By doing so, they reinstate an immune system that actively targets cancer cells, representing a critical advance in the treatment of advanced NSCLC [2]. Remarkably, these medications have demonstrated unprecedented enhancements across various clinical efficacy measures for patients with locally advanced or metastatic NSCLC [3]. Compared with traditional chemotherapy drugs, ICI improves patient survival outcomes while causing fewer adverse reactions, and has become an integral part of treatment algorithms [4].
Fatty liver disease, resulting from imbalanced metabolism due to unhealthy dietary habits and irregular lifestyles, has emerged as the most prevalent chronic liver disease worldwide [5]. Currently, non-alcoholic fatty liver disease (NAFLD) affects approximately 25% of the global adult population, with a particularly high incidence of 27% among Asian populations [6]. NAFLD is not just a liver disease, but it is also closely linked to systemic metabolic disorders and immune system abnormalities. Recent investigations have suggested that NAFLD is associated with alterations in the immune microenvironment, potentially influencing immune responses [7]. Patients with NAFLD often have comorbidities such as obesity, hypertension, and diabetes, which are known to be associated with immune dysfunction. There are also studies suggesting that the liver microenvironment may play a key role in NAFLD. The liver provides a unique pro-inflammatory microenvironment composed of a variety of immunoreactive cells, including Kupfer cells (KCs), T cells, antigen-presenting cells (APC), and hepatic stellate cells (HSC) [8]. These cells possess the capacity to augment the immune response and expedite the progression of liver fibrosis and damage. In addition to this, these immune cells secrete various bioactive factors, such as pro-inflammatory cytokines, leptin, bile acids, and free fatty acids, which effectively interact with the constituents of the liver microenvironment, precipitating inflammation, fibrosis, and lipid toxicity. The coexistence of metabolic dysfunction caused by NAFLD and alterations in the gut microbiota may collaborate to stimulate innate and adaptive immune responses, ultimately promoting both hepatic and systemic inflammatory reactions [9]. Notably, a recent study demonstrated that NAFLD could attenuate the efficacy of immunotherapy in hepatocellular carcinoma [10]. In this study, a preclinical model of hepatocellular carcinoma induced by non-alcoholic steatohepatitis was employed as the experimental model. Utilizing therapeutic immunotherapy aimed at PD1, a notable augmentation of activated CD8( +) PD1( +) T cells within the tumor milieu was observed. However, despite this immune response, tumor regression did not occur, implying a compromised functionality of tumor immune surveillance. Previous research has explored the relationship between NAFLD and the immune system, prompting consideration of whether NAFLD impacts immune checkpoint inhibitor treatment. To date, no studies have assessed the impact of NAFLD on the real-world efficacy outcomes of first-line ICI therapy for NSCLC in routine clinical practice. Therefore, our retrospective study aims to investigate the disparity in real-world treatment efficacy of ICIs in patients with and without NAFLD.
Methods and materials
Prior to the start of the study, our study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Institutional Review Board No. S054).
Study participants and design
This was a single-center retrospective cohort study. From March 2020 to July 2022, we included 479 with NSCLC who received immunotherapy at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. All participants enrolled in this study were (a) histologically or cytologically confirmed NSCLC; (b) diagnosed with stage III or IV NSCLC following NCCN Clinical Practice Guidelines in Oncology: NSCLC (Version 3.2022); [11] (c) older than 18 years of age; (d) received at least three cycles of immunotherapy; (e) diagnosed and treated at our institution. Participants for both groups were excluded if they (a) had other types of tumors; (b) received surgical treatment; (c) had other liver diseases (e.g., alcoholic fatty liver, alcoholic hepatitis, liver fibrosis, cirrhosis and drug-induced liver injury); (d) with history of excessive drinking (> 210 g/week for men and > 140 g/week for women in the past 12 months); [12] (e) whose medical record is incomplete. (f) were not initially treated with immunotherapy. Before the onset of the study, approval from the Ethics Committee of the Tongji Medical College of Huazhong University of Science and Technology. Before the onset of the study, approval from the Institutional Ethics Committee and informed consent from each subject were obtained.
Data acquisition and analysis
All CT images were obtained using a Philips Healthcare scanner. The acquisition settings included a tube voltage of 120kVp, with the tube current automatically regulated. The images were reconstructed with a matrix size of 512 × 512 and a collimation of 64 × 0.625 mm. The reconstructed slice thickness and interval were both set at 1.5 mm. To assess the CT values of the tissues, a measurement tool integrated within the Picture Archiving and Communication System (PACS) was utilized.
Two experienced radiologists (YL and BXG) performed measurements in the right lobe of the liver and at the location of the spleen at the same level, and then delineated round regions of interest (About 120mm2), avoiding bile ducts, blood vessels and artifacts (Supplementary Fig. 1). The measurements were made by the two aforementioned radiologists, and the average of the measurements was used as the final result. According to whether the calculated Liver/Spleen density ratio (L/S ratio) was less than 1, the patients were divided into patients with NAFLD and non-NAFLD. Density ratio is considered to be a reliable imaging index for the diagnosis of NAFLD [13]. Fifty patient images were randomly selected and segmented on the designated slice by a radiologist (YL) after a two-week interval, and another radiologist (GFZ) with 31 years of experience independently performed the same task to assess the repeatability of feature extraction both within and between observers. The intraclass correlation coefficient (ICC) was subsequently employed to evaluate the agreement in imaging evaluation within and between observers. The obtained ICC value of 0.94 indicates minimal differences among individuals within the group. The observers were blinded to all clinical data, and any discrepancies were resolved through consensus.
Patient outcomes
Patients’ baseline demographics and clinical outcomes were collected retrospectively. The radiological response following treatment with ICIs was assessed as per RECIST criteria v1.1 [14] on CT performed. Overall survival (OS) was defined as the time from the first dose to death or last follow-up. Progression-free survival (PFS) was defined as the time from the first dose to radiographic imaging progression or death, whichever occurred first. The Overall response rate (ORR) included patients with complete response (CR) and partial response (PR). The Disease control rate (DCR) included all patients who achieved CR, PR, or stable Disease (SD). Progressive disease (PD) included all patients with radiographic evidence of intrapulmonary progression or extrapulmonary spread.
Statistical analysis
For relevant analysis, patients were divided into two cohorts. The principle we followed was as follows: L/S ratio was defined as the CT value of the spleen divided by the CT value of the liver. Patients were divided into those with L/S ratio of less than or equal to 1 (NAFLD) and those with L/S ratio of more than 1 (Non-NAFLD). Baseline characteristics were compared within the divided L/S ratio (NAFLD vs. Non-NAFLD). The χ2 test was used to compare categorical data and the unpaired student t-test for continuous data. DCR and ORR were compared using Fisher’s exact test. To eliminate redundant and unstable features, ICC calculation and Pearson’s correlation analysis were conducted.
We employed the Kaplan–Meier method to conduct time-to-event analysis for OS and PFS. The OS and PFS were compared between patient cohorts with and without non-alcoholic fatty liver disease (NAFLD) using the log-rank test. A p-value of less than 0.05 was considered statistically significant. To further evaluate the impact of NAFLD on patient survival, univariate and multivariate Cox regression models were constructed. Parameters with a p-value less than 0.10 in the univariate analysis were included in the multivariate Cox regression model to calculate the hazard ratio (HR) and its corresponding 95% confidence interval (95% CI). Furthermore, we conducted exploratory subgroup analyses based on clinically relevant covariates and reported HRs with their respective 95% CIs within each subgroup.
Results
Baseline characteristics
Among the 479 patients with advanced NSCLC (361 NAFLD, 118 Non-NAFLD) included in this analysis, the median age was 64 years (IQR, 58–69 years), and the median BMI was 22.23 kg/m2 (IQR, 20.08–24.22 years). The baseline characteristics of patients are shown in Table 1. No significant differences in gender (p = 0.064), age (p = 0.780), diabetes (p = 0.289), hypertension (p = 0.560) and so on.
Rates of NAFLD were similar between the gender groups (p = 0.064). Two hundred and twenty-two participants (46.3%) were 65 years or older at the time of enrollment, and two hundred and fifty-seven participants (53.7%) younger. Turns out, NAFLD occurred in comparable proportions between younger and older individuals (p = 0.780). Patients with NAFLD had a higher median BMI (median 22.895 vs. 22.090 kg/m2, p < 0.001) than patients without NAFLD, which is common sense. Besides, participants with NAFLD also had higher total bilirubin (mean 12.8 vs. 10.5, p < 0.001).
Efficacy
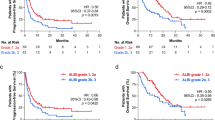
At the time of analysis, 128 (26.7%) patients had died. Because of the short follow-up, the median survival time was not reached. The median follow-up time was 22 m (IQR, 17–29 m). Survival curves were generated based on the available data. There was no statistically significant difference in PFS between NAFLD and non-NAFLD groups (p = 0.783), as was OS (p = 0.851) (Fig. 1, Fig. 2).
All patients were assessed for tumor response according to RECIST v1.1 criteria. Patients with NAFLD had similar ORR (38.1% vs. 44.9%, p = 0.199) and DCR (94.0% vs.92.0%, p = 0.452) compared to patients without NAFLD (Supplementary Table 1). NAFLD did not have a significant effect on PFS in univariate analysis (HR 0.959, 95% CI 0.713–1.292, p = 0.785) (Table 2). OS was also consistent between the NAFLD cohorts (HR 1.039, 95% CI 0.694–1.558, p = 0.851) (Table 3). Age is an important factor affecting the efficacy of treatment, and the efficacy of older patients is lower than that of younger patients. In addition, our results validated that the low short-term response and survival rates in lung cancer patients are related to the stage of lung cancer at initial diagnosis [15]. Surprisingly, the presence of hypertension was observed to be associated with a higher OS (HR 1.471 95%CI 1.018–2.127, p = 0.040). Besides, based on multivariate analysis, lactic dehydrogenase was associated with PFS (HR 1.001 95% CI 1.000–1.002, p = 0.037) and OS (HR 1.002 95%CI 1.001–1.003, p < 0.001) (Table 2, Table 3).
In addition, the preliminary subcomponent analysis was carried out in order to explore the factors that could affect the curative effect (Supplementary Fig. 2, Supplementary Fig. 3). Unsurprisingly, we found no potentially positive results in the subgroup for PFS and OS.
Discussion
This study has profound implications in the field of immunotherapy for lung cancer. The use of ICIs has become one of the most promising methods in the field of cancer treatment and has achieved satisfactory results in the clinical application of a variety of cancers [16]. Currently, due to its clear survival benefit, immunotherapy has become a standard first-line treatment modality for advanced NSCLC [17]. The population of patients with NAFLD is large, and the incidence is rising. Similarly, the proportion of lung cancer patients with underlying non-alcoholic fatty liver disease will grow. In our study, we chose the liver-spleen ratio as the assessment criterion for NAFLD because, firstly, the liver/spleen density ratio, measured with the use of CT scan, is a reliable, accurate, and noninvasive diagnostic tool for NAFLD [18]. It is considered to provide a useful noninvasive method for the detection and follow-up of patients with fatty liver when liver biopsy is contraindicated, [19] and it has been widely used in many studies [20, 21] and clinics. Second, CT-based liver/spleen density ratio is easier to measure than liver biopsy and MRI techniques. Especially in patients with lung cancer, almost every patient with lung cancer will undergo routine CT scan before treatment, which will scan the level of liver and spleen, and the liver/spleen density ratio can be easily obtained, which is practical and operable for clinicians. In the occurrence and progression of NAFLD, the immune system plays an integral role. [22] NAFLD has been shown to reduce tumor infiltration by immune cells in order to impair the ability of immunosuppressants to suppress tumor growth [23]. A recent study from the German Cancer Research Center showed that large numbers of abnormal CD8/PD-1 double-positive T cells accumulate in the liver in response to nonalcoholic steatohepatitis. If PD-1/L1 inhibitors activate these T cells, they will not only fail to kill the tumor but also aggravate liver tissue damage, making the therapeutic effect counterproductive [10]. They speculate that the same mechanism may occur in patients with other tumor types. A study by Pinto et al. showed that NAFLD may act as a potential factor affecting the immuno-tumor microenvironment in hepatocellular carcinoma, but different causes cause different responses. [24] However, a meta-analysis concluded otherwise [25]. On the contrary, we demonstrated NAFLD delivers no clinical benefit for patients with NSCLC who undergo ICI-based therapy, which is consistent with Zhou et al.’s research [26]. In addition, we validated that NAFLD did not have an impact on the survival time of patients in a larger cohort analysis. We speculated that NAFLD may induce changes in the components of the local immune microenvironment of the liver, aggravate liver injury and the progression of liver cancer, [27] but has a weak effect on the systemic immune system, which can only lead to a decline in the efficacy of immunotherapy in liver cancer, but the same performance cannot be seen in lung cancer. Nevertheless, complex regulatory mechanisms for the interaction of NAFLD with the immune system have not been fully defined, which is expected to be investigated in future studies.
At baseline, we found that NAFLD patients tended to have high total bilirubin and BMI. Generally, the proinflammatory mechanisms of NAFLD trigger high levels of total bilirubin [28]. The relationship of NAFLD and BMI mapped its relationship with obesity, which is complicated. Excess adiposity is often considered to be a manifestation of metabolic disorders associated with chronic systemic inflammation. Especially in the tumor microenvironment, it can cause an imbalance of the immune system [29]. Previous studies have shown that higher BMI seems to be associated with improved OS in patients with NSCLC after immunotherapy [30]. However, in univariate analysis, BMI was not verified to influence clinical benefit in our study. A recent mate analysis [31] shows that there is no evidence to prove a straight causal relationship between obesity and outcomes of ICIs, but sarcopenic obesity is a predictor of poor survival outcomes and toxicity in cancer patients receiving chemotherapy [32]. This may be because the parameters of body composition analysis are complex and need to be taken into consideration by multiple factors.
After performing multivariate analysis, we discovered that older patients predicted shorter survival, while patients with stage IV cancer tended to have a worse prognosis than those with stage III cancer, which is a well-known conclusion. The ECOG performance score (ECOG PS) is a widely used method for physicians to comprehensively assess patients’ symptoms and mobility. The higher the ECOG PS, the worse the patient’s physical condition. We observed that ECOG PS can predict the prognosis of patients with non-small-cell lung cancer treated with immune checkpoint inhibitors, consistent with Miao et al. [33] Patients with hypertension may have higher OS in patients after immunotherapy, which may be related to the immune-inflammatory mechanism of hypertension onset. Previous studies have shown that hypertension induces the increase of extracellular ATP, which induces the enhancement of APC and T cell function [34]. An alternative perspective focuses on prior treatment of hypertension. The drug α2 adrenoceptor agonists used in the treatment of hypertension can mediate the function of tumor-associated macrophages and CD4( +) T cells so that hypertensive patients with a history of related treatment can better benefit from immunotherapy [35]. Interestingly, lactate dehydrogenase appears to be associated with both long- and short-term survival in lung cancer patients. Our data suggested that baseline lactate dehydrogenase levels may be associated with the response to immunotherapy. Mezquita et al. [36] propose in a multicenter study that baseline lactate dehydrogenase levels are a marker of response to immunotherapy in patients with NSCLC. Many later studies have also reached similar conclusions, indicating that LDH may be an easily available indicator for predicting immune efficacy and has value in the stratified treatment of patients.
Our study has some limitations. First and foremost, this is a retrospective study subject to collection and selection bias. Although we can easily evaluate the L/S ratio on a plain CT scan of the lung, CT as a noninvasive assessment of NAFLD has relatively poor performance in detecting mild steatosis and quantitative steatosis [37]. In addition, the L/S ratio is not a gold standard for NAFLD diagnosis, liver biopsy data are needed to confirm whether NAFLD affects the efficacy of NSCLC immunotherapy ultimately. Additionally, although it represents the safety of immunotherapy, we did not record immune-related adverse events. Despite these limitations, to the best of our knowledge, this is the largest study assessing the efficacy of Immune checkpoint inhibitor therapy in patients with and without NAFLD. Therefore, prospective multi-center clinical trials are required to verify our results. In conclusion, NAFLD holds no clinical benefit for advanced NSCLC patients who undergo ICI-based treatment, but it is associated with improved outcomes in patients with LMs according to Zhou et al.’s study [26]. At the same time, we also look forward to relevant research in the future to support it.
Conclusion
In summary, our study shows NAFLD does not affect immunotherapy efficacy in NSCLC patients treated with ICI.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. If someone wants to request the date, he can contact the corresponding author by email, upon reasonable request.
References
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25(15):4592–602.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9(6):620.
Agosti P, Sabbà C, Mazzocca A. Emerging metabolic risk factors in hepatocellular carcinoma and their influence on the liver microenvironment. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):607–17.
Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3(12):1596–607.
Pfister D, Nunez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–6.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530.
Group of Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Doctor Association, Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. 2018;26(3):195–203.
Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, et al. Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology. 2011;258(3):760–6.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11(10):1653–71.
Rosato PC, Wijeyesinghe S, Stolley JM, Nelson CE, Davis RL, Manlove LS, et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat Commun. 2019;10(1):567.
Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, et al. Efficacy and Safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856–64.
Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–12.
Bydder GM, Chapman RW, Harry D, Bassan L, Sherlock S, Kreel L. Computed tomography attenuation values in fatty liver. J Comput Tomogr. 1981;5(1):33–5.
Öcal O, Ingrisch M, Ümütlü MR, Peynircioglu B, Loewe C, van Delden O, et al. Prognostic value of baseline imaging and clinical features in patients with advanced hepatocellular carcinoma. Br J Cancer. 2022;126(2):211–8.
Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab. 2019;104(5):1855–65.
Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77(4):1136–60.
Heinrich B, Brown ZJ, Diggs LP, Vormehr M, Ma C, Subramanyam V, et al. Steatohepatitis impairs T-cell-directed immunotherapies against liver tumors in mice. Gastroenterology. 2021;160(1):331-45.e6.
Pinto E, Meneghel P, Farinati F, Russo FP, Pelizzaro F, Gambato M. Efficacy of immunotherapy in hepatocellular carcinoma: Does liver disease etiology have a role? Dig Liver Dis. 2024;56(4):579–88.
Meyer T, Galani S, Lopes A, Vogel A. Aetiology of liver disease and response to immune checkpoint inhibitors: An updated meta-analysis confirms benefit in those with non-viral liver disease. J Hepatol. 2023;79(2):e73–6.
Zhou J, Zhou F, Chu X, Zhao J, Wu Y, Zhao W, et al. Non-alcoholic fatty liver disease is associated with immune checkpoint inhibitor-based treatment response in patients with non-small cell lung cancer with liver metastases. Transl Lung Cancer Res. 2020;9(2):316–24.
Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592(7854):444–9.
Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18–35.
** HR, Wang J, Wang ZJ, ** MJ, **a BH, Deng K, et al. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. 2023;16(1):103.
Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6(4):512–8.
Indini A, Rijavec E, Ghidini M, Tomasello G, Cattaneo M, Barbin F, et al. Impact of BMI on Survival outcomes of immunotherapy in solid tumors: A Systematic Review. Int J Mol Sci. 2021;22(5):2628.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Miao K, Zhang X, Wang H, Si X, Ni J, Zhong W, et al. Real-world data of different immune checkpoint inhibitors for non-small cell lung cancer in China. Front Oncol. 2022;12:859938.
Zhao TV, Li Y, Liu X, **a S, Shi P, Li L, et al. ATP release drives heightened immune responses associated with hypertension. Sci Immunol. 2019;4(36):eaau6426.
Zhu J, Naulaerts S, Boudhan L, Martin M, Gatto L, Van den Eynde BJ. Tumour immune rejection triggered by activation of alpha2-adrenergic receptors. Nature. 2023;618(7965):607–15.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–7.
Nogami A, Yoneda M, Iwaki M, Kobayashi T, Honda Y, Ogawa Y, et al. Non-invasive imaging biomarkers for liver steatosis in non-alcoholic fatty liver disease: present and future. Clin Mol Hepatol. 2023;29(Suppl):S123–35.
Acknowledgements
The authors thank everyone who participated in this project and helped with this project.
Funding
This study was conducted without any external funding support.
Author information
Authors and Affiliations
Contributions
Conception and design: Yi Li and Guofeng Zhou; Administrative support: Guofeng Zhou, Lian Yang and Feng Pan; Collection of data: Chao Chen and Shanshan Jiang; Data sorting and management: Jiyu Song; Data analysis and interpretation: Yusheng Guo, Yi Li and Weiwei Liu; Manuscript writing: Yi Li, Bingxin Gong and Yusheng Guo; Responsible for the overall content: Guofeng Zhou; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving humans were approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Institutional Review Board No. S054), and informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1 Radiological response evaluated per RECIST criteria version 1.1 stratified according to the presence or absence of nonalcoholic fatty liver disease. Supplementary Figure 1 Sample figure of the measurement of the liver/spleen ratio. A) A woman, 53 years old, did not suffer from non-alcoholic fatty liver disease. B) A man, 50 years old, suffered from non-alcoholic fatty liver disease. Supplementary Figure 2 Forest plot of progression-free survival. Supplementary Figure 3 Forest plot of overall survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Gong, B., Guo, Y. et al. Non–small cell lung cancer and immune checkpoint inhibitor therapy: does non-alcoholic fatty liver disease have an effect?. BMC Cancer 24, 535 (2024). https://doi.org/10.1186/s12885-024-12295-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12295-6