Abstract
Background
The effect of Glucocorticoids (GCs) on the treatment of Guillain-Barré syndrome (GBS) has been controversial. There is no information on whether specific subtypes of GBS respond differently to GCs. In this setting, we aimed to discuss whether GCs treating yield different effects in the distinct subtypes (acute inflammatory demyelinating polyneuropathy, AIDP; acute motor axonal neuropathy, AMAN). And further, we analyzed the impact of different doses on the outcome.
Methods
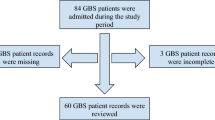
Medical records of 448 patients with a diagnosis of classic GBS admitted to 31 tertiary hospitals, located in 14 provinces of Southern China, from 1 January 2013 to 30 September 2016, were retrospectively collected. And 251 patients treated with GCs alone (AIDP=189, AMAN=62) were reviewed and analyzed.
Results
After GCs treatment, the Hughes score of AIDP patients was significantly lower than that of AMAN patients at discharge (P=0.005) and 3 months after onset (P<0.001). Further analysis revealed that among AIDP patients, the high-dose group had significantly shorter hospital stay (P=0.023), lower Hughes score at nadir (P<0.001), at discharge (P=0.005), and 3 months after onset (P<0.001), compared with the low-dose group. However, for AMAN patients, the outcome difference between groups was nonsignificant.
Conclusion
Our data suggest that the high doses of GCs may result, at least in part, from the side of the duration of hospital stay and short-term outcome, favorable outcomes in AIDP patients. Therefore, we cannot completely deny the priority of GCs in the treatment of GBS, because the effect of different doses of GCs varies in treating different subtypes. More studies are needed in the future to further validate this issue.
Trial registration
ChiCTR-RRC-17014152. Registered 26 December 2017- Retrospectively registered.
Similar content being viewed by others
Introduction
Guillain-Barré syndrome (GBS) is an immune-mediated acute peripheral neuropathy involving mainly spinal nerve roots, peripheral nerves and cerebral nerves, and is currently the most common cause of acute flaccid paralysis worldwide. As an autoimmune disease (AID) with a high rate of mortality and disability, immunotherapy is essential [1, 2].
Glucocorticoids (GCs) is considered as the most commonly used drug for the treatment of AID worldwide because of its cost-effectiveness and strong immunosuppressive effect [3]. Unfortunately, its use in GBS patients is controversial [4, 5]. Clinical trials in Europe and North America did not observe significant efficacy of GCs alone in GBS, however, as it currently stands, the actual efficacy of GCs may be underestimated, because these above-mentioned studies did not discuss the efficacy of GCs in different subtypes in a categorical manner, and the use and dosage of GCs were not uniform [6,7,8]. Scholars such as Hughes have suggested that patients with GBS with conduction block respond well to GCs, while the use of GCs in patients with denervation delays the recovery of GBS, although the specific mechanism needs to be further explored [9].
According to neuroelectrophysiological studies, GBS consists of two major subtypes, acute inflammatory demyelinating polyneuropathy (AIDP), and acute motor axonal neuropathy (AMAN) [10]. AIDP is associated with macrophage and CD4+ T cell-mediated inflammation and peripheral nerve demyelination, whereas AMAN is mainly associated with the involvement of ganglioside autoantibodies and complement [11]. Given that these two major subtypes have different pathological characteristics and pathogenesis, and their epidemiology in Asia differs from foreign studies, it is necessary to explore the mechanism of action and effects of GCs based on different subtypes.
Methods
Patient ascertainment
This is a retrospective multicenter study and the medical records of consecutive hospitalized patients with a diagnosis of GBS in 31 representative tertiary hospitals, located in 14 provinces in southern China, between 1 January 2013 and 30 September 2016, were collected. Patients who fulfilled the established clinical criteria of Asbury and Cornblath (1990) were enrolled [12]. In addition, the patients whose clinical presentation and ancillary data were typical of GBS except for preservation or exaggeration of reflexes were also included. Details regarding clinical data extraction and analysis, including inclusion and exclusion criteria, were described in our previous study [Information Extraction Information on age, sex, preceding events, initial symptoms, concomitant symptoms, severity at admission, at nadir, at discharge, length of hospitalization, findings of electrodiagnosis (EDX), treatment regime, types and doses of GCs were extracted. The motor function deficits of included patients were assessed by the Hughes Functional Grading Scale, a widely accepted scale of disability for GBS (grade 6, dead; grade 5, requiring assisted respiration; grade 4, bed-bound; grade 3, able to walk with aid; grade 2, able to walk independently; grade 1, minimal signs and symptoms, able to run; grade 0, normal) [14]. Details regarding clinical data extraction were described in our previous study [17]. A study used a rabbit model of the axonal form of GBS initially explored the reasons for the ineffectiveness of GCs in treating AMAN, suggesting that MPS did not reduce complement C3 deposition and sodium (Nav) channel disruption, but significantly reduced macrophage infiltration in the ventral roots and thus delay the axonal regeneration [18]. Studies of pathophysiology about AMAN have shown that the invasion of macrophages was rare at the acute progressive phase but significantly more frequent at the site of inflammation mainly during the recovery phase, which suggested a role for macrophages in the clearance of damaged myelin and axon fragments and promoting nerve repair and regeneration [19]. Whereas, the classical experimental autoimmune neuritis (EAN) model, which highly replicates human AIDP in terms of clinical manifestations, immunology, histopathology, and electrophysiology [20], indicated that “Classically” activated (M1) macrophages mainly accumulated at the acute phase of EAN and promoted the inflammatory response, while during the recovery phase, macrophages could change their expression profile, M2 macrophages attenuated inflammation and promoted tissue repair [21, 22]. Ultrastructural studies showed that macrophage-mediated nerve injury was a pathological hallmark of AIDP/EAN [21,22,23]. Macrophages (M1) were involved in this process by regulating cytokines, chemokines, adhesion molecules, nitric oxide (NO) and matrix metalloproteinases (MMPs), and as major antigen-presenting and effector cells, macrophages played a key role in EAN pathogenesis by expressing antigens and promoting Th1 and Th17 polarization [24]. In summary, we hypothesize that the different mechanisms of macrophages' role in the inflammatory response of AIDP and AMAN may lead to different effects of GCs therapy. We will further test our hypothesis through animal experiments. As a multicenter study, we derived relatively powerful results, but there exist inevitably some limitations. First, as a retrospective study, the long-term follow-up information was insufficient to further explore the prognosis of patients with different subtypes treated with different doses of GCs, further studies were anticipated; Second, the number of patients with AMAN subtypes in this study was relatively small; Third, because the study was a retrospective review of medical records and database, extracting bias was unavoidable. However, in order to reduce the bias as much as possible, a unified parameter standard in the analysis of NCS was adopted and data were extracted by our team members through strict training.
Conclusion
In conclusion, our data firstly provides information about whether the responses to GCs differ between the principal subtypes of GBS, and prompts recommendations about the design of future GBS trails. GCs induce different effects in specific GBS subtypes, among which high-dose GCs therapy has a better prognosis for patients with AIDP. The effects of GCs on GBS subtypes should be discussed separately in future clinical trials to explore its mechanism of action and provide more timely and effective treatment measures for GBS patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GCs:
-
Glucocorticoids
- GBS:
-
Guillain-Barré syndrome
- AIDP:
-
Acute inflammatory demyelinating polyneuropathy
- AMAN:
-
Acute motor axonal neuropathy
- AMSAN:
-
Acute motor and sensory axonal neuropathy
- AID:
-
Autoimmune disease
- MPS:
-
Methylprednisolone
- SD:
-
Standard deviation
- EAN:
-
Experimental autoimmune neuritis
- NO:
-
Nitric oxide
- MMPS:
-
Matrix metalloproteinases
References
van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–82. https://doi.org/10.1038/nrneurol.2014.121.
Sipilä JOT, Soilu-Hänninen M, Ruuskanen JO, Rautava P, Kytö V. Epidemiology of Guillain-Barré syndrome in Finland 2004–2014. J Peripher Nerv Syst. 2017;22(4):440–5. https://doi.org/10.1111/jns.12239.
Straub RH, Cutolo M. Glucocorticoids and chronic inflammation. Rheumatology (Oxford).2016;55(suppl 2):ii6–14. https://doi.org/10.1093/rheumatology/kew348.
Hughes RA. Treatment of Guillain-Barré syndrome with corticosteroids: lack of benefit? Lancet. 2004;363(9404):181–2. https://doi.org/10.1016/S0140-6736(03)15367-6.
Mastaglia FL. Neuromuscular disorders: molecular and therapeutic insights. Lancet Neurol. 2005;4(1):6–7. https://doi.org/10.1016/S1474-4422(04)00946-9.
Walgaard C, Lingsma HF, Ruts L, van Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology. 2011;76(11):968–75. https://doi.org/10.1212/WNL.0b013e3182104407.
Double-blind trial of intravenous methylprednisolone in Guillain-Barré syndrome. Guillain-Barré Syndrome Steroid Trial Group. Lancet. 1993;341(8845):586–90.
van Koningsveld R, Schmitz PI, Meché FG, Visser LH, Meulstee J, van Doorn PA, Dutch GBS study group. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barré syndrome: randomized trial. Lancet. 2004;363(9404):192–6. https://doi.org/10.1016/s0140-6736(03)15324-x.
Hughes RA, Brassington R, Gunn AA, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2016;10(10):CD001446. https://doi.org/10.1002/14651858.CD001446.pub5.
Malek E, Salameh J. Guillain-Barre Syndrome. Semin Neurol. 2019;39(5):589–95. https://doi.org/10.1055/s-0039-1693005.
Fan X, Zhang H, Cheng Y, Jiang X, Zhu J, ** T. Double Roles of Macrophages in Human Neuroimmune Diseases and Their Animal Models. Mediators Inflamm. 2016;2016:8489251. https://doi.org/10.1155/2016/8489251.
Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27 Suppl:S21–4. https://doi.org/10.1002/ana.410270707.
Liu S, **ao Z, Lou M, Ji F, Shao B, Dai H, et al. Guillain-Barré syndrome in southern China: retrospective analysis of hospitalised patients from 14 provinces in the area south of the Huaihe River. J Neurol Neurosurg Psychiatry. 2018;89(6):618–26. https://doi.org/10.1136/jnnp-2017-316930.
Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2(8093):750–3. https://doi.org/10.1016/s0140-6736(78)92644-2.
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–66. https://doi.org/10.1002/cncr.21847.
Okumura T, Kawada JI, Tanaka M, Narita K, Ishiguro T, Hirayama Y, et al. Comparison of high-dose and low-dose corticosteroid therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect Chemother. 2019;25(5):346–50. https://doi.org/10.1016/j.jiac.2019.01.003.
Cheng Q, Wang DS, Jiang GX, Han H, Zhang Y, Wang WZ, et al. Prospective study of clinical epidemiology of Guillain-Barré syndrome in Harbin, China. J Neurol Sci. 2003;215(1–2):63–9. https://doi.org/10.1016/s0022-510x(03)00187-4.
Wang YZ, Lv H, Shi QG, Fan XT, Li L, Yi Wong AH, Hao YL, Si CP, Li CL, Yuki N. Action mechanism of corticosteroids to aggravate Guillain-Barré syndrome. Sci Rep. 2015;5:13931. https://doi.org/10.1038/srep13931.
Susuki K, Rasband MN, Tohyama K, Koibuchi K, Okamoto S, Funakoshi K, Hirata K, Baba H, Yuki N. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007;27(15):3956–67. https://doi.org/10.1523/JNEUROSCI.4401-06.2007.
Gonsalvez DG, Fletcher JL, Yoo SW, Wood RJ, Murray SS, **ao J. A Simple Approach to Induce Experimental Autoimmune Neuritis in C57BL/6 Mice for Functional and Neuropathological Assessments. J Vis Exp. 2017;129:56455. https://doi.org/10.3791/56455.
Shen D, Chu F, Lang Y, Geng Y, Zheng X, Zhu J, Liu K. Beneficial or Harmful Role of Macrophages in Guillain-Barré Syndrome and Experimental Autoimmune Neuritis. Mediators Inflamm. 2018;2018:4286364. https://doi.org/10.1155/2018/4286364.
Hartung HP, Schäfer B, Heininger K, Stoll G, Toyka KV. The role of macrophages and eicosanoids in the pathogenesis of experimental allergic neuritis. Serial clinical, electrophysiological, biochemical and morphological observations. Brain. 1988;111(Pt 5):1039–1059. https://doi.org/10.1093/brain/111.5.1039.
Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol. 2001;64(2):109–27. https://doi.org/10.1016/s0301-0082(00)00060-5.
Han R, **ao J, Zhai H, Hao J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflammation. 2016;13(1):97. https://doi.org/10.1186/s12974-016-0559-x.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Independent scientific research project of Wuhan University (2042020kf0053) and the National Natural Science Foundation of China (81971055).
Author information
Authors and Affiliations
Contributions
ZX, JG and ZL took part in validating the diagnosis and information extraction; YL and JY involved in data collection and analysis, LM and SL contributed to the study design, data collection and analysis, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the Renmin Hospital of Wuhan University and conducted in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived by the ethics committee of the Renmin Hospital of Wuhan University because the analysis was retrospective.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, L., Liu, S., **ao, Z. et al. Comparison of the effects of different doses of Glucocorticoids on distinct subtypes of Guillain-Barré syndrome in Southern China. BMC Neurol 22, 46 (2022). https://doi.org/10.1186/s12883-022-02567-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02567-8