Abstract
Background and Aims
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have beneficial effects in heart failure (HF), including reverse remodelling, but the mechanisms by which these benefits are conferred are unclear. Inflammation is implicated in the pathophysiology of heart failure (HF) and there are some pre-clinical data suggesting that SGLT2 inhibitors may reduce inflammation. There is however a lack of clinical data. The aim of our study was to investigate whether improvements in cardiac remodelling caused by dapagliflozin in individuals with type 2 diabetes (T2D) and left ventricular hypertrophy (LVH) were associated with its effects on inflammation.
Methods
We measured C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin 6 (IL-6), and interleukin 10 (IL-10) and neutrophil-to-lymphocyte ratio (NLR) in plasma samples of 60 patients with T2D and left ventricular hypertrophy (LVH) but without symptomatic HF from the DAPA-LVH trial in which participants were randomised dapagliflozin 10 mg daily or placebo for 12 months and underwent cardiac magnetic resonance imaging (CMR) at baseline and end of treatment. The primary analysis was to investigate the effect of dapagliflozin on inflammation and to assess the relationships between changes in inflammatory markers and LV mass and global longitudinal strain (GLS) and whether the effect of dapagliflozin on LV mass and GLS was modulated by baseline levels of inflammation.
Results
Following 12 months of treatment dapagliflozin significantly reduced CRP compared to placebo (mean difference of -1.96; 95% CI -3.68 to -0.24, p = 0.026). There were no significant statistical changes in other inflammatory markers. There were modest correlations between improvements in GLS and reduced inflammation (NLR (r = 0.311), IL-1β (r = 0.246), TNF-α (r = 0.230)) at 12 months.
Conclusions
Dapagliflozin caused a significant reduction in CRP compared to placebo. There were correlations between reductions in inflammatory markers including IL-1β and improvements in global longitudinal strain (but not reduced LV mass). Reductions in systemic inflammation might play a contributory role in the cardiovascular benefits of dapagliflozin.
Trial registration
Clinicaltrials.gov NCT02956811 (06/11/2016).
Similar content being viewed by others
Introduction
Several large clinical trials involving patients with or without T2D have shown that SGLT2 inhibitors improve cardiovascular outcomes in individuals with heart failure or at risk of heart failure [1, 2]. While the cardiovascular benefits of SGLT2 inhibitors have been consistently demonstrated, the mechanisms by which these benefits are conferred remain unclear. One postulated mechanism is that SGLT2 inhibitors might cause beneficial cardiac remodelling, directly improving cardiovascular structure and function, such as reducing left ventricular hypertrophy (LVH) and improving systolic function [3].
LVH is a marker of cardiovascular risk that is an independent risk factor for future development of heart failure [4]. We and others have previously shown that SGLT2 inhibitors can reduce LV mass compared to placebo, and this might explain some of their cardiovascular benefit in at-risk patients [5]. In the DAPA-LVH trial, we found that 12 months of treatment with dapagliflozin significantly reduced LV mass and improved systolic function measured by GLS compared to placebo in patients with T2D and LVH without symptomatic heart failure. [6, 7]
SGLT2 inhibitors also have cardiometabolic benefits, causing weight loss and improving blood pressure [8]. These favourable changes could lead to a reduction in systemic inflammation that is associated with hypertension and obesity. CRP, a surrogate marker of inflammation, is strongly associated with poor cardiovascular outcomes [9, 10]. Beyond CRP, increased expression of various inflammatory cytokines has been associated with adverse cardiac remodelling including left ventricular hypertrophy, fibrosis, and development of heart failure [11, 12]. Higher levels of systemic inflammation are also associated with adverse outcomes in patients with heart failure. [13, 14]
Several studies have demonstrated that increased levels of inflammation are linked to LVH [15,16,17]. At a molecular level, elevation in TNF-α triggers the overexpression of pro-inflammatory molecules such as IL-6, a molecular mediator implied in up-regulation of collagen and development of LVH [18,19,20]. SGLT2 inhibitors have been shown to reduce inflammatory activation. In a preclinical study using a pharmacological model of inflammation in macrophages isolated from patients with diabetes, SGLT2 inhibitors significantly reduced the TNF-α and IL-1β [21].
Given these relationships, it is plausible that at least some of the beneficial cardiac effects of SGLT2 inhibitors might be related to an anti-inflammatory effect, however, this has not been examined in a clinical study in detail. The aim of this study was to evaluate improvements left ventricular remodelling caused by dapagliflozin were associated with its anti-inflammatory effects in patients with T2D and LVH.
Methods
Study cohort
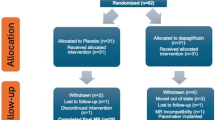
The design of DAPA-LVH trial, as well as the study design, protocol and the primary outcomes, have been published previously [22, 23]. In brief, in the trial 66 patients with T2D, LVH, and without uncontrolled hypertension or symptomatic heart failure (i.e. Stage B HF) were randomised to receive either dapagliflozin 10 mg once daily (n = 32) or placebo (n = 34) for 12 months. DAPA-LVH trial received approval from the East of Scotland Research Ethics Committee (16/ES/0131), and all participants provided written informed consent before enrolment. The trial duration spanned 10–12 months. The primary outcome was a change in absolute left ventricular mass from baseline, assessed by cardiac magnetic resonance imaging. Patients also underwent 2-dimensional transthoracic echocardiography including assessment of GLS.
Inflammatory marker analysis
Blood samples were obtained at the baseline and 12-month (primary outcome) visits. Samples were centrifuged at 3000 rpm for 10 min then the plasma was carefully decanted into aliquot and subsequently stored in -80⁰C. Plasma levels of CRP were measured using Luminex Human Discovery Assay (1-Plex) LXSAHM-01 and levels of inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-10 were determined using R&D Systems Luminex kits. All experimental measurements were performed according to the manufacturer’s instructions and repeated in duplicate. A full blood count was also obtained, and the eosinophil to lymphocyte ratio (ELR), and neutrophil to lymphocyte ratio (NLR) were measured as an additional markers of systemic inflammation.
Statistical analyses
Normally distributed continuous variables were reported as mean ± standard deviation while non-normally distributed continuous variables were reported as median with interquartile ranges in parentheses (IQR). Categorical variables were reported as number with percentage of the sample in parentheses. To determine whether differences between the dapagliflozin and placebo groups, a t–test was used for normally distributed continuous variables, a Mann-Whitney U test was used for non-normally distributed continuous variables, and a chi-square test was used for categorical variables. An analysis of covariance (ANCOVA) was used to determine whether dapagliflozin caused a significant change in CRP, cytokine levels, ELR, and NLR compared to placebo, adjusting for baseline CRP, cytokine levels, and NLR. We evaluated the correlation between changes in inflammation and changes in LV mass and GLS using the Spearman correlation coefficient. Finally, to determine whether the beneficial effects of dapagliflozin on cardiac remodelling were related to baseline levels of inflammation we divided the cohort by the median level of each inflammatory marker and assessed the effect of dapagliflozin on LVM and GLS, testing for the interaction between groups.
Results
Baseline characteristics
60 participants from DAPA-LVH had available plasma samples at baseline and 12 months, matched with echo and CMR studies. The baseline characteristics of the individuals are shown in Table 1. The mean age of the cohort was 65 years and 60% were male. The median CRP was 1.07 mg/L and there were no statistically significant differences in participants randomised to dapagliflozin or placebo. Baseline characteristics stratified by the median CRP at baseline are presented in Supplementary Table 1. Patients with higher levels of CRP were more likely to be female and have a history of stroke, lower LV mass, and have a lower prevalence of hypertension and were less likely to be prescribed angiotensin receptor blockers and statins.
Effects of dapagliflozin on inflammatory markers
After 12 months of treatment, dapagliflozin significantly reduced CRP compared to placebo (Table 2). The change in CRP in the dapagliflozin group was 1.07 ± 0.61 mg/L vs. placebo group 3.04 ± 0.59 mg/L; p = 0.026), leading to an absolute mean difference of -1.96 (95% confidence interval (CI): -3.68 to -0.24, p = 0.026). Dapagliflozin did not cause any significant changes in TNF-α, IL-1β, IL-6, IL-10, ELR, or NLR compared to placebo.
Association between changes in inflammation and left ventricular mass and function
Overall, after 12 months of treatment, compared to placebo, dapagliflozin caused a significant reduction in LV mass (change in LVM: dapagliflozin − 4.61 ± 0.89 g vs. placebo − 0.87 ± 0.86 g; mean difference − 3.74 g, 95% CI: -6.24 to -1.24; p = 0.004) and improvement in GLS (change in GLS: dapagliflozin − 1.63% ± 0.44% vs. placebo group − 0.31% ± 0.45%; mean difference − 1.32%, 95% CI: -2.59% to -0.04%; p = 0.043).
The correlation between change in inflammatory makers at 12 months and change in LV mass and GLS at 12 months are summarised in Table 3. In summary, there were no significant correlations between changes in LV mass at 12 months and changes in CRP (r = 0.124) or any other inflammatory markers. However, our study showed a modest relationship between changes in GLS at 12 months and changes in NLR (r = 0.311), TNF-α (r = 0.230), and IL-1β (r = 0.246).
Impact of baseline levels of inflammation on the effect of dapagliflozin on left ventricular mass and systolic function
When the cohort was stratified by the median CRP at baseline there was no significant difference in the effect of 12 months treatment with dapagliflozin on LV mass. In individuals with CRP < 1.07 mg/L, dapagliflozin caused a reduction in LV mass regardless of baseline CRP (mean difference in LV mass with dapagliflozin vs. placebo – CRP < 1.07 mg/L: -5.87 g; 95% CI: 1.19 to -8.32 g, p = 0.037; CRP ≥ 1.07 mg/L: -2.47 g; 95% CI: -6.77 to 1.82 g, p = 0.247; interaction p value = 0.58) (Table 4) (Fig. 11). Results were similar with other inflammatory markers, demonstrating no significant interaction between baseline levels of inflammation and the effect of dapagliflozin on LV mass (Table 4) (Fig. 1). There was also no significant interaction between baseline CRP level and the effect of dapagliflozin on GLS, with dapagliflozin causing similar improvements in GLS regardless of CRP at baseline (mean difference in GLS with dapagliflozin versus placebo – CRP < 1.07 mg/L: -1.12%; 95% CI: -2.83–0.58%, p = 0.188; CRP ≥ 1.07 mg/dl: -1.65%; 95% CI: -3.95–0.63%, p = 0.144; interaction p value = 0.77) (Table 4) (Fig. 2). Furthermore, there was no significant interaction between baseline levels of TNF-α, IL-6, IL-10, ELR, or NLR and the effect of dapagliflozin on GLS (Table 4). Although the interaction p value was not statistically significant, there did appear to be a signal that individuals with higher baseline levels of IL-1β might have a greater improvement in GLS with dapagliflozin compared to those with lower levels (mean difference with dapagliflozin versus placebo – IL-1β above median (0.49 pg/ml): -2.61%; 95% CI: -4.17% to -1.04%, p = 0.002; IL-1β below median: -0.26%; 95% CI: -2.03–1.50%, p = 0.757; interaction p value = 0.13) (Fig. 2).
Treatment and 12 months change in left ventricular mass stratified by above and below the median of baseline CRP of 1.07 mg/L, baseline IL-1β of 0.49 pg/ml, baseline IL-6 of 1.26 pg/ml, and baseline NLR of 2.06 in DAPA-LVH trial. Data are presented as mean [95% confidence interval (CI)]. Change in LV mass in grams in the placebo group (black) and the dapagliflozin group (blue)
Treatment and 12 months change in global longitudinal strain (GLS) stratified by above and below the median of baseline CRP of 1.07 mg/L, baseline IL-1β of 0.49 pg/ml, baseline IL-6 of 1.26 pg/ml, and baseline NLR of 2.06 in DAPA-LVH trial. Data are presented as mean [95% confidence interval (CI)]. Change in GLS in percentage in the placebo group (black) and the dapagliflozin group (blue)
Discussion
In this study, we made several findings. We found that, in patients with T2D and stage B HF, dapagliflozin did significantly reduce CRP compared to placebo, however, it did not lead to significant changes in other markers of inflammation. We also found that the reduction in LV mass caused by dapagliflozin was similar irrespective of baseline levels of inflammation and that changes in LV mass after 12 months of treatment were not correlated with changes in inflammation. In contrast, we did find modest correlations between reductions in NLR, TNF-α, and IL-1β, and improvement in GLS. These results suggest that while any anti-inflammatory effects of dapagliflozin are perhaps not central to its mechanism of benefit, it is possible that reductions in levels of inflammation may contribute to preventing LV systolic impairment, particularly in patients with higher levels of systemic inflammation.
We found a consistent benefit of dapagliflozin on LV mass reverse remodelling regardless of baseline levels of inflammation. This has been reported in other studies, although we have now extended this finding to include other inflammatory markers and CRP. In post hoc analysis of the EMPA-HEART CardioLink-6 trial that included a total of 97 patients with T2D and coronary artery disease (CAD), the effect of empagliflozin on LV mass was stratified by NLR of > 2.1 to evaluate the correlation between baseline NLR and cardiac remodelling. The authors found that the reduction in LV mass caused by empagliflozin was independent of baseline NLR [24], which is consistent with our finding showed that the reduction in LV mass caused by dapagliflozin was not significantly different in patients with T2D and stage B HF, regardless of baseline level of CRP or other inflammatory cytokines. Our study included individuals without CAD and investigated more inflammatory markers CRP, and cytokines, in addition to NLR.
Bozkurt et al. [25] showed an association between elevation of circulating inflammatory makers and deterioration in left ventricular systolic function, finding that there was a stronger correlation between severity of HF and TNF-α or IL-6 levels than with CRP, which was similar to our findings. CRP is a marker of inflammation that is downstream of these cytokines, and it is possible that more upstream markers of inflammation may have more impact on HF outcomes. This hypothesis is also supported by our finding that changes in NLR, a marker of systemic inflammation (as opposed to just one specific pathway) were also more strongly associated with GLS than CRP.
Increased levels of inflammatory cytokines are linked to a reduction in systolic function [26]. Inhibition of IL-1β has been demonstrated to be associated with improvement in LV strain [27] and reduction in heart failure hospitalisation [28]. In our study, we found that reduced IL-1β was correlated with improved subclinical LV systolic function (GLS). Evidence from other studies suggests that this cytokine in particular may be targets for treatment in HF patients, with a post-hoc analysis of CANTOS demonstrating that the IL-1β inhibitor canakinumab caused a dose-dependent reduction in subsequent HF hospitalisation in individuals with high levels of inflammation at randomisation [28]. Although we did not demonstrate a statistically significant reduction in IL-1β with dapagliflozin in our study, we may have been underpowered to do so. Our results are in line with other studies suggesting that IL-1β might be a treatment target for prevention of HF.
Limitations
Our study is limited by a relatively small sample size and therefore may be underpowered. The study also did not include all inflammatory markers related to cardiovascular disease though we investigated the most common cytokines and markers of inflammation implicated in heart failure.
Conclusions
In our analysis of the DAPA-LVH trial, we found that in patients with T2D and LVH dapagliflozin significantly reduced CRP but did not cause statistically significant changes in inflammatory cytokines, eosinophils, neutrophils, ELR, and NLR. Although the reduction in LV mass induced by dapagliflozin remained consistent irrespective of the baseline levels of inflammation, there was a modest relationship between higher levels of inflammation at 12 months and worse systolic function. These findings, suggest that while the potential anti-inflammatory effects of dapagliflozin are perhaps not the primary driver of its beneficial mechanism, there is a possibility that these anti-inflammatory effects may contribute to some of the cardiovascular benefits of dapagliflozin, playing a role in prevention of left ventricular systolic impairment, especially in patients with elevated levels of systemic inflammation.
Data availability
The data used and/or analysed during the current study are available from the corresponding author on request.
Abbreviations
- ANCOVA:
-
An analysis of covariance
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CMR:
-
cardiac magnetic resonance imaging
- CRP:
-
C-reactive protein
- GLS:
-
Global longitudinal strain
- HF:
-
Heart failure
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- LV:
-
Left ventricle
- LVH:
-
Left ventricular hypertrophy
- TNF-α:
-
Tumor necrosis factor alpha
- NLR:
-
Neutrophil-to-lymphocyte ratio
- SGLT2:
-
Sodium-glucose co-transporter 2
- T2D:
-
type 2 diabetes
References
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with Cardiovascular and kidney outcomes in patients with type 2 diabetes: a Meta-analysis. JAMA Cardiol. 2021;6(2):148–58.
Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400(10354):757–67.
Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–9.
Moura B, Aimo A, Al-Mohammad A, Keramida K, Ben Gal T, Dorbala S, et al. Diagnosis and management of patients with left ventricular hypertrophy: role of multimodality cardiac imaging. A scientific statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2023;25(9):1493–506.
Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of Empagliflozin on Left Ventricular Mass in patients with type 2 diabetes Mellitus and Coronary Artery Disease: the EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140(21):1693–702.
Brown A, Gandy S, Mordi IR, McCrimmon R, Ramkumar PG, Houston JG, et al. Dapagliflozin improves left ventricular myocardial longitudinal function in patients with type 2 diabetes. JACC Cardiovasc Imaging. 2021;14(2):503–4.
Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41(36):3421–32.
Lopaschuk GD, Verma S. Mechanisms of Cardiovascular benefits of Sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632–44.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43.
Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–91.
Takei Y, Di Tullio MR, Homma S, Boden-Albala B, Rundek T, Sacco RL, et al. Soluble tumor necrosis factor receptor 1 level is associated with left ventricular hypertrophy: the northern Manhattan study. Am J Hypertens. 2009;22(7):763–9.
Rosello-Lleti E, Rivera M, Martinez-Dolz L, Gonzalez Juanatey JR, Cortes R, Jordan A, et al. Inflammatory activation and left ventricular mass in essential hypertension. Am J Hypertens. 2009;22(4):444–50.
Mooney L, Jackson CE, Adamson C, McConnachie A, Welsh P, Myles RC, et al. Adverse outcomes Associated with Interleukin-6 in patients recently hospitalized for heart failure with preserved ejection fraction. Circulation Heart Fail. 2023;16(4):e010051.
Curran FM, Bhalraam U, Mohan M, Singh JS, Anker SD, Dickstein K, et al. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021;8(4):3168–79.
Masiha S, Sundstrom J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens. 2013;27(1):13–7.
Fang L, Ellims AH, Beale AL, Taylor AJ, Murphy A, Dart AM. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. 2017;9(11):5063–73.
Karayigit O, Nurkoc SG, Celik MC. Systemic immune-inflammation index (SII) may be an effective indicator in predicting the left ventricular hypertrophy for patients diagnosed with hypertension. J Hum Hypertens. 2023;37(5):379–85.
Lillo R, Graziani F, Franceschi F, Iannaccone G, Massetti M, Olivotto I, et al. Inflammation across the spectrum of hypertrophic cardiac phenotypes. Heart Fail Rev. 2023;28(5):1065–75.
Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem. 2012;287(4):2666–77.
Zhao L, Cheng G, ** R, Afzal MR, Samanta A, Xuan YT, et al. Deletion of Interleukin-6 attenuates pressure overload-Induced Left ventricular hypertrophy and dysfunction. Circ Res. 2016;118(12):1918–29.
Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127.
Brown AJ, Lang C, McCrimmon R, Struthers A. Does dapagliflozin regress left ventricular hypertrophy in patients with type 2 diabetes? A prospective, double-blind, randomised, placebo-controlled study. BMC Cardiovasc Disord. 2017;17:1–12.
Brown A, Gandy S, Mordi IR, McCrimmon R, Ramkumar PG, Houston JG, et al. Dapagliflozin improves left ventricular myocardial longitudinal function in patients with type 2 diabetes. Cardiovasc Imaging. 2021;14(2):503–4.
Verma R, Moroney M, Hibino M, Mazer CD, Connelly KA, Yan AT, et al. Baseline neutrophil-to-lymphocyte ratio and efficacy of SGLT2 inhibition with empagliflozin on cardiac remodelling. ESC Heart Fail. 2023;10(3):2127–33.
Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15(4):331–41.
Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the multi-ethnic study of atherosclerosis. Eur Heart J. 2010;31(7):875–82.
Ikonomidis I, Tzortzis S, Lekakis J, Paraskevaidis I, Andreadou I, Nikolaou M, et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart. 2009;95(18):1502–7.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with Canakinumab for the Prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–99.
Acknowledgements
The authors express gratitude to the trial participants, the dedicated staff members of the Division of Clinical and Molecular Medicine at the University of Dundee, as well as the team at the Immunoassay Biomarker Core Laboratory and the Clinical Research Centre, University of Dundee, Ninewells Hospital, and Medical School in the United Kingdom.
Funding
The Dapa-LVH trial was funded by an Externally Sponsored Research grant from AstraZeneca (grant number ESR-14-1016). This analysis was funded by Tenovus Scotland (T20-58). Dr Dihoum is a Libyan government funded clinical research fellow. IRM is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/ICRF/24/26101).
Author information
Authors and Affiliations
Contributions
A.D. conceived the study, performed the principal analysis for this study, and wrote the initial draft. I.R.M. also conceived the study and supervised the initial analysis and manuscript preparation. A.J.B., R.J.M., and C.C.L. provided critical revision, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the ethical standards as laid down in the 1964 declaration of Helsinki and was approved by the East of Scotland Research Ethics Committee (16/ES/0131). All participants provided informed consent for all study-related activities including future research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12872_2024_4022_MOESM1_ESM.docx
Supplementary Material 1: Supplementary Table 1-: Baseline characteristics stratified by median CRP (1.07 mg/L) in the DAPA-LVH trial
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dihoum, A., Brown, A.J., McCrimmon, R.J. et al. Dapagliflozin, inflammation and left ventricular remodelling in patients with type 2 diabetes and left ventricular hypertrophy. BMC Cardiovasc Disord 24, 356 (2024). https://doi.org/10.1186/s12872-024-04022-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04022-7