Abstract
Background
The formation of shoots plays a pivotal role in plant organogenesis and productivity. Despite its significance, the underlying molecular mechanism of de novo regeneration has not been extensively elucidated in Capsicum annuum ‘Dempsey’, a bell pepper cultivar. To address this, we performed a comparative transcriptome analysis focusing on the differential expression in C. annuum ‘Dempsey’ shoot, callus, and leaf tissue. We further investigated phytohormone-related biological processes and their interacting genes in the C. annuum ‘Dempsey’ transcriptome based on comparative transcriptomic analysis across five species.
Results
We provided a comprehensive view of the gene networks regulating shoot formation on the callus, revealing a strong involvement of hypoxia responses and oxidative stress. Our comparative transcriptome analysis revealed a significant conservation in the increase of gene expression patterns related to auxin and defense mechanisms in both callus and shoot tissues. Consequently, hypoxia response and defense mechanism emerged as critical regulators in callus and shoot formation in C. annuum ‘Dempsey’. Current transcriptome data also indicated a substantial decline in gene expression linked to photosynthesis within regenerative tissues, implying a deactivation of the regulatory system governing photosynthesis in C. annuum ‘Dempsey’.
Conclusion
Coupled with defense mechanisms, we thus considered spatial redistribution of auxin to play a critical role in the shoot morphogenesis via primordia outgrowth. Our findings shed light on shoot formation mechanisms in C. annuum ‘Dempsey’ explants, important information for regeneration programs, and have broader implications for precise molecular breeding in recalcitrant crops.
Similar content being viewed by others
Introduction
Shoot formation is a central topic in both plant science and agricultural biotechnology. It is important for several key processes, notably the generation of transgenic plants and the propagation of desired plants. From shoot formation on the pluripotent callus, plants reproduce tissues such as stems, roots, and leaves during vegetative propagation; thus, each organ forms a new plant. The success of whole plant regeneration largely depends on understanding when and how shoots develop from the proliferating cells of explants. Shoot formation is a complex process driven by specific genetic networks and molecular pathways of auxin and cytokinin [1,2,3,4,5,6]. Pluripotency, derived from somatic cells, is pivotal for the formation of all plant organs throughout a plant’s life cycle. Notably, stem cells found in the shoot apical meristem (SAM) and root apical meristem (RAM) play vital roles, with their functions defined by their location. The totipotency of leaf tissues allows somatic embryos to form from individual leaf cells or cell groups, revisiting early developmental stages in mature plants [5, 7]. Therefore, delving into the molecular underpinnings of shoot formation provides rich insights into plant growth, reproduction, survival, and adaptability.
Phytohormone-related genes are critical for SAM development in callus tissue. In in vitro tissue cultures, phytohormones regulate transcription factors such as CUP-SHAPED COTYLEDON (CUC), which responds to auxin and brassinosteroid (BR), and WUSCHEL (WUS), which is activated by high cytokinin and low auxin conditions. Overexpression of these transcription factors leads to somatic embryos for shoot formation on pluripotent callus tissue [3,4,5, 8]. PIN-FORMED 1 (PIN1), an auxin efflux carrier, is instrumental in new SAM formation by regulating auxin accumulation [1, 4, 5]. The ethylene-related genes ETHYLENE OVERPRODUCER 1 (ETO1) and ETHYLENE RESPONSE 1 (ETR1) also influence shoot formation. This is likely linked to auxin and cytokinin responses, based on explant sensitivity to ethylene signaling [6]. ETHYLENE RESPONSE FACTOR 109 (ERF109) contributes to tissue repair and organ formation by regulating stem cell activity and auxin production [9, 10], and ERF109-mediated responses are tightly controlled by multiple phytohormones like ethylene (ET), abscisic acid (ABA), and jasmonic acid (JA) [11]. Therefore, a crosstalk of phytohormones centered around auxin within a genetic network plays a pivotal role in plant morphogenesis.
Auxin flow is a significant factor in shoot formation and post-embryonic organogenesis, including the formation of new leaves, flowers, and lateral roots. Plant organogenesis, highlighted by local auxin accumulation at the initiation sites of emerging organs, creates distinct phyllotactic patterns essential for organized plant growth [4, 12,13,14]. The concentrations and ratios of auxin and cytokinin determine specific developmental pathways in the SAM during shoot growth [4,5,6, 15]. Furthermore, auxin responses are related to the onset of plasmodesmata production in the cell wall, marking a commitment to regeneration [2]. The physiological role of auxin during organ formation also encompasses the differentiation of new vasculature, including leaf venation [13, 16].
Plant organogenesis comprises fine-tuned developmental processes. Pinpointing the core regulatory system for shoot formation during the developmental processes ensures proper and efficient schemes for plant regeneration. A genome-wide association study (GWAS) of shoot formation using 190 natural Arabidopsis accessions reported that a smaller set (∼ 5%) of identified genes serve as master regulators that are crucial under multiple procedures and traits [15]. A comparative transcriptomic study within Solanaceae—including tomato, potato, petunia, pepper, tobacco, and Nicotiana benthamiana—showed a high degree of sequence conservation and species-specific transcripts even though these six species represent diverse phenotypes for different agronomic purposes [17]. Both petunia and Arabidopsis are known as representative regenerating species [18], whereas bell pepper is treated as a recalcitrant species. Thus, it is crucial to consolidate the essential genetic factors by considering distinct genetic backgrounds across species.
Capsicum annuum ‘Dempsey’ is a sweet and bell pepper having virus- and bacterial spot-resistant traits that originates from a three-way cross between the ‘PI163192′, ‘PI264281′, and ‘Jupiter’ cultivars [19,20,21]. The ‘Dempsey’ cultivar is highlighted as an excellent genetic resource with multiple disease resistances, a non-functional pun1 allele as a standard of non-pungency [22], and available whole genomic information [23]. Besides serving as crop feed, bell peppers are also a great source of antioxidants, especially carotenoids; phenolic compounds; and vitamins A, C, and E [20, 21, 24]. ‘Dempsey’ is genetically distinguishable from other cultivars within the sweet pepper group by comparative analysis among plastome sequences [25] and displays different cellular properties in its polyethylene glycol (PEG)-mediated CRISPR/Cas9 ribonucleoprotein (RNP) delivery compared to hot pepper [26, 27]. Various attempts to apply genome-editing tools to ‘Dempsey’ and other peppers have overcome challenges related to genetic transformation, regeneration, and the study of molecular function in pepper [3, 21, 22, 27,28,29]. However, studying the molecular mechanism of shoot formation, which is essential for pepper functional genetics, precise molecular breeding, and biotechnology applications, is lacking. The knowledge acquired from studying shoot development in bell peppers can lead to pepper genetic manipulation improving quality and increasing yields.
Herein, we first report on comparative transcriptomic analyses of the ‘Dempsey’ cultivar to identify critical shoot-forming genes expressed during plant regeneration. The comparison included contrasting transcriptomes of callus and shoot segments induced from leaf tissue. Next, we aimed to uncover the genetic orchestration underlying shoot development from leaf explants in the ‘Dempsey’ cultivar, particularly focusing on phytohormones, by comparing a wide range of developmental gene expression profiles across five intra- and inter-family species: ‘Dempsey’ pepper, three petunias, and Arabidopsis. We also identify vital genes in callus-to-shoot organogenesis using gene expression profiling with enriched pathways. Our findings elucidate novel components of the shoot-forming mechanism and highlight potential genetic markers that can be instrumental for pepper transformation and molecular breeding, with practical applications in future agricultural biotechnology.
Materials and methods
Plant materials
The bell pepper C. annuum ‘Dempsey’ was provided by the Vegetable Breeding Research Center (VBRC) in Seoul, Republic of Korea. For RNA isolation and library preparation, 5-week-old or 18-week-old C. annuum ‘Dempsey’ fully expanded leaf tissues near the shoot apical bud were used as the WT. ‘Dempsey’ callus and shoot were prepared using the previously described method [21]. For generating callus and shoot tissues, 1 cm of young apical leaves from 5-week-old C. annuum ‘Dempsey’ plants were placed on a shoot induction medium (SIM), and the produced tissues were collected after four weeks. The explants were dissected using a surgical scalpel under a stereo microscope, frozen using liquid nitrogen, and finely ground with a mortar and pestle. The resulting finely ground tissue powder was stored at -80℃ for further experiments.
Total RNA isolation
Total RNA was isolated from ‘Dempsey’ leaf, callus, and shoot tissue using Tri-RNA Reagent (Favorgen, FATRR 001), and RNA concentrations were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA; ND-2000). Each RNA sample’s quality was checked using a bioanalyzer (Agilent Technologies, Santa Clara, CA, USA; 2100 Bioanalyzer), and the RNA integrity number (RIN) was confirmed to be above 8.
RNA-seq library preparation
Following the manufacturer’s protocol, RNA-seq libraries were constructed from two biological replicates using a TruSeq Stranded mRNA Library Prep Kit (Illumina, San Diego, CA, USA; RS-122-2101). They were constructed using the indexed adaptors provided in the kit and pooled for sequencing. Sequencing with a paired 2 × 75 bp length was performed on the Illumina MiSeq platform. The paired-end raw sequencing reads were cleaned (satisfying Q20 and Q30 ≥ 80%) and adaptors were trimmed using the CLC Genomics Workbench v20.0.4 (Liu & Di 2020). The quality of trimmed reads was checked using the FastQC program [30]. All cleaned RNA-seq libraries were deposited at NCBI under BioProject accession number PRJNA1063381.
Map** of RNA-seq reads and abundance estimation
The clean reads of each RNA-seq library were mapped using the HI-SAT2 program [31] and the C. annuum ‘Dempsey’ genome assembly ASM2707356v1 (NCBI accession number: GCA_027073565.1) [23]. The ‘Dempsey’ transcripts were annotated by running the BLAST program with the transcripts of Petunia axillaris, Petunia exserta, and Petunia integrifolia, and the NCBI datasets of C. annuum ‘Zunla-1’ (accession number GCF_000710875.1), C. annuum ‘UCD-10X-F1’ (accession number GCF_002878395.1), Capsicum baccatum ‘PBC81’ (accession number GCA_002271885.2), and A. thaliana TAIR10.1 (accession number GCF_000001735.4), with an e-value cut-off of 1e − 3 [32,33,34]. C. baccatum and Capsicum chinense were mapped to C. baccatum ‘PBC81’ genome assembly ASM227188v2 [3, 34]. To estimate the abundance of the annotated transcripts, we used the featureCounts tool with the default parameters [35]. We conducted the steps from map** to quantification on the Galaxy platform [36]. All data on the expression (raw and TMM-normalized counts) and annotation of C. annuum ‘Dempsey’ transcripts with homologs are provided in Data S1.
Differential expression analysis
A differentially expressed gene (DEG) analysis was conducted in two steps using edgeR and NOISeq packages [37, 38]. First, based on the Benjamini and Hochberg’s approach, the DEG analysis was performed using the edgeR package with a false discovery rate (FDR) of less than 0.05. The low-expression genes were filtered out, and the Trimmed Mean of M-values (TMM)-normalized counts were obtained based on Counts Per Million (CPM) value of more than 1 in at least one sample. Second, non-DEGs were filtered using the NOISeq package with a ranking score (RS) of 20.1, based on an Euclidean distance of the fold change (FC) of more than 2 and an absolute expression difference (D) greater than 20 between the TMM-normalized counts [38, 39]. After filtering non-DEGs, a heatmap was generated, and the subsequent DEGs were clustered into six K-means clusters using Morpheus software (https://software.broadinstitute.org/morpheus). The distance metric for clustering was the Pearson correlation coefficient. For understanding correlations across RNA-seq samples, the R function prcomp was used for performing the PCA analysis, the R packages ggplot2 was used to visualize the PCA plot, and the R package corrplot was used to perform the Pearson’s correlation efficient analysis and visualize the correlation matrix [40,41,42].
Functional enrichment analysis
To elucidate the shoot formation mechanism and gene expression in ‘Dempsey’, we annotated DEGs based on the gene ontology for specifying biological processes (GO: BP) database using the R package org.At.tair.db [43]. To visualize the results, a dot-plot and cnetplot were produced using the R package clusterProfiler v4.0, hel** us understand the primary biological processes involved and identify hub genes within the K-means clusters [44].
Comparative transcriptome analysis
For comparing the DEGs of C. annuum ‘Dempsey’, we selected and downloaded DEG datasets from RNA-seq data containing of ‘Seedling’, ‘Callus’, and ‘Shoot apices’ data for P. axillaris, P. exserta, and P. integrifolia [32], Additionally, we downloaded A. thaliana RNA-seq data containing CON0d [control, after-cutting < 15 mins], CIM4d [on callus-inducing media for 4 days], and SIM4d and SIM6d [on shoot-inducing media for 4 and 6 days, respectively] data, representing gene expression datasets derived from wild-type, callus, and shoot tissue [45]. To identify DEGs common among the similar tissue types of all five species (including our ‘Dempsey’ data), we used the DiVenn 2.0 program and InteractiVenn programs [46, 47]. We focused on callus and shoot-specific gene regulation by identifying DEGs between leaf/seedling and callus and leaf/seedling and shoot RNA-seq samples, thereby discerning upregulated and downregulated genes in the callus and shoot tissues compared to basal control. In the comparative datasets, we identified all DEGs using an FDR < 0.05 in ‘Dempsey’ and A. thaliana and a probability value (q) of > 0.8 in Petunia spp., using a previously described method [39]. The Arabidopsis Hormone Database 2.0 (AHD2.0) and the R package org.At.tair.db were utilized for analyzing the relationships between phytohormones and DEGs in the five species [43, 48].
Chlorophyll content measurement
To confirm the deactivation of the regulatory system governing photosynthesis and chlorophyll biosynthesis, chlorophyll content was measured using a 96-well microplate and methanol extraction [49]. After measuring the fresh weight (FW) of the tissue powder, chlorophyll extraction was achieved by adding 1 mL of methanol and vortexing for 2 min. Following extraction, samples were centrifuged for two minutes at 16,760 g, and the supernatant was separated from the pellet and added to an empty 2 mL Eppendorf tube. The pellet underwent a second extraction using 1 mL of methanol and further voltexing for 2 min. After centrifuging at 16,760 g, the supernatant was transferred, the pellet was discarded, and the two supernatants were combined to measure the chlorophyll content. Chlorophyll content was calculated using Warren’s (2008) formula and normalized using the FW of the tissue powder [49].
Quantitative real-time reverse-transcription PCR (qRT-PCR)
To validate the RNA-seq data of ‘Dempsey’, the expression profiles of genes were examined by qRT-PCR. The cDNAs were synthesized using the ReverTra Ace qPCR RT Master Mix with a gDNA Remover kit (Toyobo, Osaka, Japan). qRT-PCR was performed with the cDNA as template using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Vilnius, Lithuania) under the following conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All reactions were performed in three biological replicates. We utilized Actin (CaDEM03G20100) and GAPDH (CaDEM03G33920) as multiple reference genes for qRT-PCR data normalization. All primer sequences for qRT-PCR are listed in Table S1. Relative gene expression was calculated using the 2−ΔΔCт method [50].
Results
‘Dempsey’ transcriptomes reveal DEGs in proliferating callus tissue and emerging shoots
To explore DEGs across distinct C. annuum ‘Dempsey’ tissues, we collected total RNA sequencing results from leaf tissue (WT), leaf explant-derived callus tissue (Callus), and callus-driven emerging buds (Shoot) (Fig. 1A). BLAST results showed that 39,392 genes in the ‘Dempsey’ genome were annotated to 22,482 genes of Capsicum annuum ‘Zunla-1’, 22,398 genes of Capsicum annuum ‘UCD-10X-F1’, and 14,130 genes of Arabidopsis thaliana. Map** rates of RNA-seq reads on the ‘Dempsey’ genome ranged from 98.34 to 98.61% (Table S2).
We identified 3,787 and 2,514 genes that were differentially expressed (at an FDR < 0.05) in callus tissue compared to ‘Dempsey’ leaf tissue (Callus vs. WT) and shoot tissue compared to ‘Dempsey’ leaf tissue (Shoot vs. WT), respectively. Of these, the 1,696 and 1,079 genes exhibited increased expression in Callus vs. WT and Shoot vs. WT, respectively. In contrast, 2,091 and 1,435 genes exhibited decreased expression in Callus vs. WT and Shoot vs. WT, respectively (Fig. 1B).
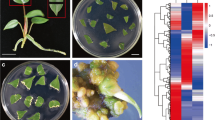
Analysis of DEGs from callus and shoot tissue in Capsicum annuum ‘Dempsey’. (A) Example images with the scale bar of samples from leaf (WT), callus, and shoot tissues used for RNA-seq analysis: ‘Dempsey’ leaf WT (left), leaf-derived callus tissue (middle), callus-derived emerging shoot tissue (right). (B) Volcano plots depicting the DEGs of callus versus WT (left) and shoot versus WT (right) comparisons. (C) Principal component analysis (PCA) plot of the TMM-normalized counts of the RNA-seq samples. (D) Correlation matrix plot (Corrplot) showing Pearson’s correlation efficient of RNA-seq samples. The filled fraction of the circle in each pie charts (upper) corresponds to the Pearson’s correlation coefficient (lower). Blue and red colors denote positive and negative correlations, respectively. (E) A heatmap of DEGs, which are grouped by K-means clustering into six clusters (colored bars) with numbers in brackets indicating the number of genes in each cluster. The X-axis represents the two biological replicates of RNA-seq samples taken from the three tissue types. The Y-axis represents individual gene expression levels, visualizing the variations in gene expression across tissue types and from the three tissue types. The Y-axis represents individual gene expression levels, visualizing the variations in gene expression across tissue types and samples. (F) Log2-transformed expression levels of genes in each K-means cluster. The X-axis represents the two biological replicates of RNA-seq samples taken from the three tissue types. The Y-axis represents the mean-centered log2 expression level of the genes. Each graph is marked by a line representing the mean log2 expression level in the color assigned to each cluster in panel E.
The transcriptomic data were simplified using a PCA analysis to better understand the relationships among the tissue type samples. As a result, the first and second principal components (PC1 and PC2) explained 64.4% and 20.0% of the total variation, respectively (Fig. 1C). Through a pairwise comparison of the RNA-seq samples, a notable difference was observed between the WT libraries and the libraries from the Callus and Shoot, most conspicuously in the PC1 (Fig. 1C). According to 20.0% of the PC2, the Pearson’s correlation coefficient of each tissue group was 85.0 in the WT and 0.98 in the Callus and Shoot, thereby representing high sample similarities in the positive correlation (Fig. 1C, D).
By applying the criteria of CPM greater than 1 in edgeR and an RS greater than 20.1 in NOISeq, excluding low-expression genes with non-expression patterns, 8,110 cluster signature genes were obtained. After filtering featureless genes, we identified 6 K-means clusters containing 552 genes in the smallest cluster and 1,819 in the largest (Fig. 1E). Cluster 1, containing 1,667 genes, predominantly showed upregulated genes associated with callus tissue; Cluster 3, containing 552 genes, predominantly showed upregulated genes associated with shoot tissue; and Cluster 5, containing 1,219 genes, showed upregulated genes associated with both callus and shoot tissues. These clusters were distinct from clusters 2, 4, and 6, which contained 1,819, 1,653, and 1,200 genes, respectively (Fig. 1E). These Clusters showed high expression levels of WT samples. Specifically, Cluster 2 and 4 displayed unique gene expression patterns for WT1 and WT2, possibly reflecting distinct biological variations. In contrast, Cluster 6 exhibited gene expression common to both WT samples, indicating a leaf-specific expression profile (Fig. 1E, F).
Gene ontology (GO) enrichment analysis reveals genetic features of callus and shoot formation
In the GO enrichment profile of callus-specific Cluster 1, cell division was highlighted during callus proliferation by the ‘cytokinesis’ and ‘mitotic cell cycle process’ annotations, and mitochondrial energy processes were exhibited in the ‘cellular respiration’, ‘energy derivation by oxidation of organic compounds’, and ‘tricarboxylic acid cycle’ annotations (Fig. 2A).
Visualization of GO terms (Y-axis) representing biological processes for K-means clusters in Capsicum annuum ‘Dempsey’. (A) Cluster 1; (B) Cluster 2; (C) Cluster 3; (D) Cluster 4; (E) Cluster 5; (F) Cluster 6. The dot color represents the adjusted p-value (p.adjust; −log10[FDR]). The dot size represents the number of DEGs representing each GO term (Count). The X-axis indicates the number of DEGs in each GO term relative to the total number of genes in each K-means cluster (GeneRatio)
The WT-specific clusters 2, 4, and 6 were enriched with genes involved in photosynthetic processes and chloroplast development, with annotations such as ‘photosynthesis’, ‘chloroplast organization’, ‘pigment metabolic process’, ‘response to light intensity’, ‘tetrapyrrole metabolic process’, ‘photosynthetic electron transport chain’, ‘reductive pentose-phosphate cycle’, and ‘carbon fixation’ (Fig. 2B, D, F).
Shoot-specific Cluster 3 was particularly enriched with genes implicated in the phytohormone-activated signaling pathway and cell differentiation during shoot formation, including annotations in ‘regulation of signal transduction’, ‘regulation of cell communication’, ‘pattern specification process’, ‘post-embryonic plant morphogenesis’, ‘regionalization’, ‘meristem maintenance’, ‘anatomical structure formation involved in morphogenesis’, ‘phloem or xylem histogenesis’, and ‘plant organ formation’ (Fig. 2C). Additionally, Cluster 3 was enriched with genes involved in the response to monosaccharides as a metabolic feature and genes responsive to pathogens, such as those in the defense responses to viruses and symbionts as biotic stressors (Fig. 2C).
Cluster 5, which comprised genes with high expression in both callus and shoot tissues, included gene annotations involved in the immune system, such as ‘RNAi-mediated antiviral immune response’; developmental processes, such as ‘xylem and phloem pattern formation’ and ‘multidimensional cell growth’ (Fig. 2E). Clusters 1 and 5 were also enriched in genes involving the protein modification and recycling for callus formation, with annotations such as ‘intracellular protein transport’, ‘protein maturation’, ‘alpha-amino acid metabolic process’, ‘proteasomal protein catabolic process’, ‘protein folding’, ‘ERAD pathway’, and ‘response to hypoxia’ (Fig. 2A, E).
Defense mechanisms and hypoxia responses are involved in callus growth and shoot formation
In clusters 1, 3, and 5, genes associated with defense mechanisms and hypoxia showed high expression levels based on the GO enrichment analysis. Thus, we investigated DEGs involved in defense and hypoxia within these clusters. Multiple defensins (CaDEM07G00190, CaDEM07G00200, CaDEM07G01550, CaDEM07G01560, and CaDEM12G07160) in clusters 1 and 5 were highly upregulated (log2 fold changes [log2|FC|] > 5.9) in ‘Dempsey’ callus tissue (Table S3). Lignin-based pathogen barrier-forming CASP-like proteins (CaDEM05G03350 and CaDEM05G03420) in clusters 1 and 5 were also highly upregulated (log2|FC| > 5.5) (Table S3). Moreover, we found 21, 4, and 5 upregulated peroxidases belonging to clusters 1, 3, and 5, respectively (Table S3). Thus, we revealed a potential link indicating that the hypoxic condition in callus tissue was due to the limitation of oxygen diffusion by respiratory bursts or lignin barriers, each acting as a defense mechanism during callus and shoot formation (Table 1). This is examined in more depth in the Discussion Sect. 4.2.
Gene concept network analysis illustrates key hub genes for callus growth and shoot formation
Using gene-concept network plots (cnetplots), we visually portrayed the intricate gene networks of clusters 1, 3, and 5, showcasing the interaction among DEGs and the top five significantly enriched GO terms pertinent to callus and shoot formation in C. annuum ‘Dempsey’ (Fig. 3). The cnetplots also exhibit ‘hub genes’, i.e., those having a high level of connectivity within the gene network among the top five significantly enriched GO terms (Fig. 3). Hub genes could be highly influential in each cluster because they may regulate or be regulated by many other genes, suggesting their importance for understanding critical regulatory mechanisms in biological processes. The interconnected representation in the plots provide a concise overview of the associations between genes and GO terms in callus-specific Cluster 1 (Fig. 3A), shoot-specific Cluster 3 (Fig. 3B), and callus/shoot-specific Cluster 5 (Fig. 3C).
Gene-concept network (Cnetplot) depicting gene-to-GO term relationships in Capsicum annuum ‘Dempsey’. (A) the callus-specific cluster (Cluster 1); (B) the shoot-specific cluster (Cluster 3); (C) the cluster representing both callus and shoot tissue DEGs (Cluster 5). The cnetplots visualize the top 5 significantly enriched GO terms and the genes related to those GO terms in each cluster (category). The size of dots at the center of each cluster represents the number of genes related to the associated GO term (size). The vertical color bar indicates the log2|fold change| in gene expression for each gene (foldChange). Red arrowheads indicate an extreme change in gene expression (log2|fold change| > 5). Blue arrowheads indicate key ternary or quaternion hub genes providing high connectivity among the morphogenesis or hypoxia-related GO terms. Black arrowheads indicate key binary hub genes providing high connectivity among the morphogenesis or hypoxia-related GO terms
In callus-specific Cluster 1, the significant increase in major biological processes indicated 27 hub genes centered around the GO term ‘response to endoplasmic reticulum (ER) stress’, which shared co-expression in the primary biological processes of ‘protein maturation’, ‘protein folding’, and ‘proteasomal protein catabolic process’ (Fig. 3A). In Cluster 1, transcription levels of the AT5G49940 homologue, the iron-sulfur cluster assembler NFU2 (CaDEM01G12270), and AT3G14250 homologues, RBR E3 ubiquitin ligase genes (CaDEM03G39660 and CaDEM03G41470), were significantly increased, with log2|FC| values of 7.9, 7.6, and 5.9, respectively, in the Callus vs. WT comparison. Co-expression of the three genes in ‘Dempsey’ was shared by the response to ER stress (Fig. 3A, Data S1).
In shoot-specific Cluster 3, prominent gene expression was observed for AT1G14760 homologue, KNATM (CaDEM06G26780), and AT1G07090 homologue, LSH6 (CaDEM05G03950), with expression log2|FC| values of 5.8 and 7.1, respectively, in the Shoot vs. WT comparison (Fig. 3B). Of the top five GO terms, two key hub genes were observed within the ternary GO network. The AT5G02030 homologue, BEL1-like homeodomain RPL (CaDEM09G08050), was the hub among GO terms ‘phloem or xylem histogenesis’, ‘post-embryonic plant morphogenesis’, and ‘pattern specification process’. The AT1G73590 homologue, auxin efflux carrier PIN1 (CaDEM03G42500), was a hub gene among ‘phloem or xylem histogenesis’, ‘post-embryonic plant morphogenesis’, and ‘regulation of signal transduction’. The roles of these two hub genes, RPL and PIN1, in Cluster 3, indicates their involvement shoot differentiation and growth in the ‘Dempsey’ cultivar. In particular, ‘pattern specification process’ and ‘post-embryonic plant morphogenesis’ GO terms are known to be associated with shoot growth (Fig. 3B).
Another ten hub genes were discovered within binary GO networks in Cluster 3 (Fig. 3B). As morphogenesis-related gene expression, ‘phloem or xylem histogenesis’- and ‘pattern specification process’-associated genes were connected with three hub genes: AT5G62940 homologue, HCA2 (CaDEM03G36060); AT1G52150 homologues, ATHB-15 (CaDEM12G18880 and CaDEM03G44680); and AT3G22810 homologue, FL2 (CaDEM03G14860). GO terms ‘pattern specification process’ and ‘post-embryonic plant morphogenesis’ were connected via the AT2G41370 homologue, BOP2 (CaDEM05G00340); AT5G49660 homologue, XIP1 (CaDEM04G21220); and AT4G37180 homologue, UIF1 (CaDEM02G29970). ‘Phloem or xylem histogenesis’ and ‘regulation of signal transduction’ were linked by the AT5G03280 homologue, EIN2 (CaDEM09G02640), an ethylene signal transduction-related gene. ‘Post-embryonic plant morphogenesis’ and ‘regulation of signal transduction’ were connected by two hub genes, AT3G63010 homologue, GID1B (CaDEM06G00810), and AT4G16340 homologue, SPK1 (CaDEM01G40660). Lastly, ‘regulatory ncRNA-mediated gene silencing’- and ‘pattern specification process’-annotated genes were linked through the AT1G48410 homologue, AGO1 (CaDEM06G26600).
In Cluster 5, sharing upregulation of both callus and shoot, three hypoxia-related GO terms were noticeably selected as top five GO terms in Cluster 5, including ‘response to oxygen levels’, ‘response to decreased oxygen levels’, and ‘response to hypoxia’ (Fig. 3C). The expression of nine genes was significantly increased in all three of these terms (log2|FC| > 5.0) in the Callus vs. WT comparison: AT1G09070 homologue, SRC2 (CaDEM08G01510), AT5G15120 homologue, PCO1 (CaDEM03G36330), AT5G54960 homologue, PDC2 (CaDEM02G17450), AT1G77120 homologue, ADH1 (CaDEM04G14000), AT5G47060 homologue, DUF581 (CaDEM06G16370), AT4G10265 homologue, WIP3 (CaDEM07G20060), AT3G43190 homologue, SUS4 (CaDEM09G25130), AT3G02550 homologue, LBD41 (CaDEM03G43310), and AT3G25882 homologue, NIMIN-2 (CaDEM03G43370) (Fig. 3C). The hypoxia response attenuator 1 (HRA1) homologue, AT3G10040 (CaDEM09G01260), was a quaternion hub gene mediating ‘response to hypoxia’, ‘response to oxygen levels’, ‘response to decreased oxygen levels’, and ‘negative regulation of gene expression’ (Fig. 3C). Thus, our analysis revealed that hypoxia may strongly influence the gene expression patterns in Cluster 5, which was associated with both callus and shoot tissues.
Comparative transcriptomic analyses of five species identified the conserved essential genes for callus and shoot development
To get mainly conserved genetic features for shoot formation in C. annuum ‘Dempsey’, we conducted a comparative analysis using DEG datasets derived from the RNA-seq data of Petunia axillaris, Petunia exserta, Petunia integrifolia, and A. thaliana.
This analysis revealed that 15 genes exhibited increased expression in callus tissue when compared to the basal control across all five species (Fig. 4A, Fig. S1A). Based on GO term profiling, three of these genes were involved in defense mechanisms (AT4G16260, OSM34, and AT5G61890), three with hypoxia (ADH1, ETR2, and AT4G19880), and one each with the development of callus (WOX13) and shoot (BRC1) tissues (Fig. 4A, Data S2). Meanwhile, 146 genes showed decreased expression in all species’ callus tissue. These were involved in broad and varied biological functions (Fig. 4A, Fig. S1B, Data S2): ten genes were related to photosynthesis (LHCB4.2, CA1, LHCA4, PSAN, PSAD-2, PORA, ALB1, PPDK, RSH1, and NARA5), ten to chloroplast organization and movement (AT1G15290, CHUP1, ALB3, AT5G67385, COL2, CDF1, GLK2, HCF106, EMB3123, and EMB1303), and seven to chlorophyll biosynthesis (PORA, CH1, GSA1, ALB1, CHLM, GLK2, and EMB1303) (Fig. 4A). These three functions implicate a reduction in photosynthetic activity in calli across all five species.
DiVenn diagrams depicting the conserved gene regulation patterns of five species: Capsicum annuum ‘Dempsey’, Petunia axillaris, Petunia exserta, Petunia integrifolia, and Arabidopsis thaliana. (A) callus tissues of five species; (B) shoot tissues of five species. Red stars indicate the upregulated genes common among the five species (5-species conserved UP), while blue squares denote the common downregulated genes (5-species conserved DOWN)
Additionally, nine genes exhibited increased expression in shoot tissues compared to the basal control across all five species (Fig. 4B, Fig. S1C). Of these, six were involved in cell differentiation and development (ANT, AT2G32280, OPS, LSH3, MP, and PHB) and one each were involved in hypoxia response (ETR2) and protein ubiquitination (AT5G48800), and one had an unknown function (EXL3) (Fig. 4B, Data S3). Among these genes, ANT plays a role in the primordial outgrowth of the shoot, LSH3 suppresses cell division in shoot organ boundaries, VCC (AT2G32280) and OPS play roles in the development of early provasculature development through auxin maxima, and ETR2 inhibits root elongation through ET. At the same time, 56 genes showed decreased expression in the shoots of all species (Fig. 4B, Fig. S1D, Data S3). These were involved in various biological functions, including photosynthesis (TAP38, ACDO1, PPDK, PORA, LHCB4.2, NdhS, CA1, RbcX2, PnsL2, LHCA4, TED4, and AT1G80380), chloroplast organization and movement (GLK2 and CHUP1), chlorophyll biosynthesis (ACDO1, GLK2, PORA, and CH1), fatty acid metabolism (CYP77A4, EFE, and AKINBETA1), cell wall biogenesis (XTH7), ER body organization (AT4G15545), photomorphogenesis and skotomorphogenesis (PORA), hypoxia response (AT5G54940), defense mechanisms and stress responses (EFE, AAE3, and CA1), and the regulation of monosaccharides or decreased response to disaccharides (TPPH, F2KP, and ACR11) (Fig. 4B).
Interestingly, ETR2, a gene associated with responses to hypoxia and ET, was the sole gene upregulated in both callus and shoot tissue (Fig. S1E, Data S2 and S3), while decreased expression of genes associated with photosynthetic regulation (TRX-M4, GLK2, and PIF4), photosynthesis (LHCB4.2, LHCA4, NdhS, PnsL2, PORA, CH1, CA1, and PPDK), chloroplast movement (CHUP1), response to sucrose (ACR11), and trehalose biosynthesis (TPPH) were shown in both callus and shoot tissue (Fig. S1F, Data S2, and Data S3). The total chlorophyll a and b content of the Callus (average in 0.14 mg/g FW) and Shoot (average in 0.22 mg/g FW) was significantly decreased compared to the WT (average in 0.58 mg/g FW), supporting a reduction in photosynthetic activity and chlorophyll biosynthesis in the callus and shoot tissues (Fig. S2). Therefore, we discovered an overall decline in photosynthesis-related gene expression in regenerative tissues, indicating a shut-down of the regulatory mechanism for photosynthesis.
A high proportion of auxin-related genes are conserved in ‘Dempsey’ for de novo shoot formation
To further investigate the significant genes influencing shoot formation in the ‘Dempsey’ cultivar, we used a comparative transcriptome analysis focusing on with phytohormone-related genes, categorizing clusters 1, 3, and 5 according to their prevalent phytohormonal relationships (Fig. 5A, B, C). The three clusters revealed distinct relationships with eight phytohormones: ABA, auxin, cytokinin (CK), ET, gibberellic acid (GA), BR, JA, and salicylic acid (SA) (Fig. 5A, B, C). Callus-specific Cluster 1 showed a notable linkage with, in descending order, the hormones ABA, auxin, JA, ET, and SA (15, 15, 13, 10, and 9 genes, respectively) (Fig. 5A, B). Shoot-specific Cluster 3 was mainly related to auxin and ABA (16 and 12 genes, respectively) (Fig. 5A, B). Cluster 5 was highly related to ABA, auxin, ET, JA, and GA (20, 16, 16, 12, and 9 genes, respectively) (Fig. 5A, B). Clusters 1 and 5 were at least marginally associated with all eight phytohormones (Fig. 5A, B), indicating that they all played differential roles in the gene expression of comparative tissues of ‘Dempsey’. In the context of de novo regeneration, focusing on the phytohormone-related genes of clusters 1, 3, and 5, the proportion of genes in Cluster 1 associated with each phytohormone category were SA (47.4%), BR (45.5%), JA (41.9%), CK (38.5%), ABA (31.9%), auxin (31.9%), ET (30.3%), and GA (18.2%) (Fig. 5C). Meanwhile, the proportion of genes in shoot-forming Cluster 3 for each phytohormone category were auxin (34.0%), CK (30.8%), BR (27.3%), ABA (25.5%), ET (21.2%), and JA (19.4%), while SA was less strongly associated (10.5%) and GA was not involved with Cluster 3 genes (Fig. 5C).
To better understand the primarily phytohormone-related genes involved in shoot formation, we investigated conserved phytohormone-related genes in five species. This showed that phytohormonal gene regulation varied in the callus and shoot transcriptomes of the five species (Fig. 5D, E). In the comparative callus transcriptomes (Fig. 5D), the grou** of clusters 1 and 5 was interpreted as relating to regulation of callus formation before shoot development (Cluster 3). In the comparative shoot transcriptomes (Fig. 5E), the grou** of clusters 3 and 5 was interpreted as regulation of shoot formation occurring after or during and after callus development, respectively.
Phytohormone-associated genes belonging to callus-specific Cluster 1 (red), shoot-specific Cluster 3 (aqua), and the cluster representing both callus and shoot tissue, Cluster 5 (pink), in C. annuum ‘Dempsey’. (A) a polar plot of phytohormone-related genes in each K-means cluster, with the eight phytohormones represented by each pole (see the light blue box for phytohormone abbreviations); (B) a stacked bar plot showing the gene numbers in each K-means cluster (colors) for each phytohormone (X-axis); (C) a proportional stacked bar plot of the genes in each K-means cluster (colors) for each phytohormone (X-axis); (D) heatmap of comparing the transcriptomes of the five species in callus tissue; (E) heatmap of comparing the transcriptomes of the five species in callus tissue. RNA-seq data were analyzed to identify phytohormone-related DEGs in each cluster with expression of C. annuum ‘Dempsey’ (red), A. thaliana (green), P. axillaris (blue), P. exserta (purple), and P. integrifolia (brown). The color scale bar of heat intensity indicates the log2-transformed fold change (log2|FC|) in expression (the grey box on the heatmap indicates no recorded expression). Red arrowheads indicate highly upregulated genes (log2|FC| > 2). The black arrowhead indicates the most upregulated gene (a log2|FC| of 1.5–2) for shoot formation (Cluster 3). The black boxes to the left of the heatmaps indicate the phytohormone(s) related to each gene
For the calli of the five species, we revealed phytohormone-related gene regulations of three genes that were conserved among the five species in Cluster 1 and seven in Cluster 5 (Fig. 5D). Of these, PR4 and GH3.1 of Cluster 1 and ERF1, AT4G17260, and MP of Cluster 5 showed prominent upregulation (log2|FC| > 2) in callus tissue (Fig. 5D, Data S4). For the shoots of the five species, we found ten genes in Cluster 3 and eight in Cluster 5. Of these, DHAR1 was the most upregulated in Cluster 3 (log2|FC| = 1.6) (Fig. 5E, Data S4) and CKX3 and MP showed prominent upregulation in Cluster 5 (log2|FC| > 2) during the shoot formation (Fig. 5E, Data S4). Thus, the MP of Cluster 5 was prominently upregulated for overall callus and shoot development (Fig. 5D, E). Multispecies phytohormone-related gene regulation showed that auxin-associated genes represented the highest proportion of genes in both callus (50%) and shoot (50%) tissues (Fig. 5D, E). Therefore, these results indicated that in all five species, including C. annuum ‘Dempsey’, de novo callus and shoot formation was primarily related to auxin (Fig. 5D, E).
To validate the accuracy of the RNA-seq transcriptomic analyses, we performed qRT-PCR for five DEGs in shoot-specific genes (ANT, MP, PIN1, LSH3, and PHB), based on our comparative transcriptome results. The qRT-PCR results corroborated the RNA-seq data, demonstrating a significant increase in the mRNA levels of all five DEGs in Shoot vs. WT (Fig. S3). Among these genes, MP, LSH3, and PHB showed considerable shifts in gene expression levels, observed in both Callus vs. WT and Shoot vs. WT (Fig. S3), reinforcing the pivotal functions that these genes play in mediating cellular differentiation processes for de novo shoot formation. This concordance between the qRT-PCR and RNA-seq data not only confirmed the reliability of our transcriptomic analyses but also underscored the critical role of auxin signaling pathways in de novo shoot development of plants.
Discussion
Allocation of energy resources during callus formation in ‘Dempsey’
A significant suppression of photosynthesis was found in the pluripotent callus of rice [51]. Similarly, a loss of chlorophyll was also observed in ‘Dempsey’ callus tissue (Fig. 1A, S2). The transcriptomic analysis showed that downregulated genes in both callus and shoot tissue outnumbered upregulated genes (Fig. 1B), signifying many negatively regulated pathways in reproductive tissues compared to leaf tissue (WT). Furthermore, the DEGs found in reproductive tissues and those in leaf tissue showed incompatible gene expression patterns, and their representative biological processes, de novo shoot formation and photosynthesis, appeared to have an antagonistic relationship as a balanced mechanism in ‘Dempsey’ (Fig. 1E, F, and 2).
The WT-specific clusters, clusters 2, 4, and 6, were enriched in genes involved in photosynthetic processes and chloroplast development, represented by GO terms such as ‘photosynthesis’, ‘chloroplast organization’, ‘pigment metabolic process’, ‘generation of precursor metabolites and energy’, ‘response to light intensity’, ‘tetrapyrrole metabolic process’, ‘photosynthetic electron transport chain’, ‘reductive pentose-phosphate cycle’, and ‘carbon fixation’ (Fig. 2B, D, F). These biological processes are common in plant gene expression analyses, being important for the coordination of photosynthetic activity, light response, and the metabolic processes needed to support energy production and growth from light [52]. Therefore, the photosynthetic processes represented in clusters 2, 4, and 6 were incompatible with the callus- and shoot-specific processes in clusters 1, 3, and 5 (Fig. 2).
As the common pattern seen in the three Petunia spp., Arabidopsis, and ‘Dempsey’, the simultaneous increase in the expression of genes for callus and shoot development and decrease in the expression of photosynthetic-related genes could indicate a strategic trade-off made by the plant (Fig. 4A, B, and S1). For instance, in situations of limited light availability, a plant might focus more on growth to reach light via skotomorphogenesis, reducing de-etiolation and maintaining high levels of photosynthesis [53]. Thylakoid modulation to regulate photosynthesis via etiolation/de-etiolation could also reflect a particular developmental stage where the plant prioritizes rapid shoot growth or a stress response leading to the redistribution of the plant’s resources based on environmental cues or challenges [54]. Thus, we consider the allocation of energy sources in plants may prioritize callus and shoot formation over energy production through photosynthesis. Reduced activities of photosynthetic genes lead to diminished adenosine triphosphate (ATP) production in the regenerative calli [55].
Recently, adenosine monophosphate (AMP), an oxidized form of ATP, has been identified as an enhancer for shoot formation on pluripotent calli [56]. Herein, the callus-specific expression of ‘Dempsey’, Cluster 1 included a large number of ‘nucleobase-containing small molecule metabolic process’-related genes, indicating that this was a noteworthy biological activity during callus formation (Fig. 2A). Hence, molecules involved in energy metabolism are thought to play a crucial role in promoting shoot formation; however, the detailed molecular mechanisms remain unknown due to inconsistent findings related to cyclic adenosine monophosphate (cAMP), adenosine diphosphate (ADP), and ATP [56]. Nevertheless, AMP might be essential for de novo shoot formation from callus tissue in ‘Dempsey’. Previous transcriptomic results from AMP-treated and CK-treated plants displayed minimal overlap, suggesting AMP is a crucial metabolite for regenerating competence in calli during tissue culture conditions associated with hypoxia [56].
New insights into the effects of hypoxia and oxidative stress on de novo callus and shoot formation
The transcriptome analyses of shoot development in C. annuum ‘Dempsey’ highlighted overall roles of stress responses. GO enrichment analyses included diverse reactions to environmental stressors, such as wounding and hypoxia, and immune activities related to pathogens and symbiont responses, all of which contribute to the plant’s overall stress responses (Fig. 2A, C, E). Moreover, the multi-species comparative transcriptome analysis indicated molecular markers of defense mechanism (PR4, GH3.1, and ERF1) and hypoxia (ADH1, ETR2, and AT4G19880) across all five species during callus formation (Figs. 4A and 5D).
In the context of defense mechanisms, PR4, a pathogenesis-related gene, functions primarily in plant defense by contributing to local acquired resistance against necrotrophic pathogens, typically through its involvement in the JA signaling pathway [57]. The gene GH3.1, an IAA-amido synthetase, regulates hormonal balance by conjugating hormones to amino acids, affecting both plant growth and defense responses [58]. The gene ERF1 integrates JA and ET signals in plants to activate defense genes against pathogens and herbivores [59]. Thus, responses to biotic stressors mediated by JA, auxin, and ET in callus tissue was conserved across all five species.
In hypoxia, cell damage creating wound tissue may induce a hypoxic condition because increased respiration by defense responses may result in oxygen depletion [60, 61]. Additionally, dense tissues lacking intercellular air spaces—such as in phloem and bulky, lignin-containing organs like seeds and fruits—may restrict oxygen flow, causing hypoxia [60]. Indeed, lignin polymerized by CASP-like proteins and peroxidases can block water and solute movement [62, 63]. Leaves accumulate lignin in response to bacterial pathogens through CASP-like proteins, forming a physical barrier similar to the Casparian strip in roots, thereby restricting pathogen spread and inhibiting their growth [62]. When lignin nanoparticles are tightly embedded in an artificial cellulose fiber membrane, the material properties show reduced oxygen permeability through the membrane [64]. Clusters 1, 3, and 5 of the ‘Dempsey’ transcriptome included genes associated with hypoxia response and defense mechanism-related lignin polymerization, such as those coding defensins, CASP-like proteins, and lignin-forming peroxidases (Table 1, Table S3). Furthermore, fungal lignin peroxidases can trigger the defense response of plants, including cell death, reactive oxygen species (ROS) bursts, callose deposition, and the upregulation of immunity-related genes [60, 66,67,68,69,70]. A previous study by Ikeuch et al. (2022) highlighted the significant role of WUSCHEL-RELATED HOMEOBOX 13 (WOX13) in controlling tissue repair mechanisms via wound stress, in regulating WIND2 and WIND3 in callus tissue formation, with RNA-seq data pointing towards hypoxia as a key factor in this process in Arabidopsis [61]. Despite AtWIND1 and AtWIND3 (AT1G78080 and AT1G36060) as downregulated and upregulated DEGs, respectively, no similar differential expression patterns of WIND transcription factors were found in Dempsey and three petunia species in our transcriptome comparison (Data S1). This lack of consistent expression patterns across five species may suggest a species-specific reliance on wound and regeneration signaling pathways, underlining the complexity of plant tissue repair mechanisms.
In addition, we showed that gene expression in Cluster 3 involved responses to monosaccharides (Fig. 2C). The accumulation of monosaccharides and sucrose in plant tissues is commonly seen as a reaction to abiotic stress. Additionally, high concentrations of monosaccharides in quickly expanding young plant structures can stimulate cell proliferation and the outgrowth of new leaves [61, 71]. A convergence of stress/defense mechanism-related genes underscores the intricate balance between reproductive development and environmental adaptability [66, 72,73,Full size image