Abstract
Background
Independent origins of carnivory in multiple angiosperm families are fabulous examples of convergent evolution using a diverse array of life forms and habitats. Previous studies have indicated that carnivorous plants have distinct evolutionary trajectories of plastid genome (plastome) compared to their non-carnivorous relatives, yet the extent and general characteristics remain elusive.
Results
We compared plastomes from 9 out of 13 carnivorous families and their non-carnivorous relatives to assess carnivory-associated evolutionary patterns. We identified inversions in all sampled Droseraceae species and four species of Utricularia, **uicula, Darlingtonia and Triphyophyllum. A few carnivores showed distinct shifts in inverted repeat boundaries and the overall repeat contents. Many ndh genes, along with some other genes, were independently lost in several carnivorous lineages. We detected significant substitution rate variations in most sampled carnivorous lineages. A significant overall substitution rate acceleration characterizes the two largest carnivorous lineages of Droseraceae and Lentibulariaceae. We also observe moderate substitution rates acceleration in many genes of Cephalotus follicularis, Roridula gorgonias, and Drosophyllum lusitanicum. However, only a few genes exhibit significant relaxed selection.
Conclusion
Our results indicate that the carnivory of plants have different effects on plastome evolution across carnivorous lineages. The complex mechanism under carnivorous habitats may have resulted in distinctive plastome evolution with conserved plastome in the Brocchinia hechtioides to strongly reconfigured plastomes structures in Droseraceae. Organic carbon obtained from prey and the efficiency of utilizing prey-derived nutrients might constitute possible explanation.
Similar content being viewed by others
Background
Carnivorous plants, also known as insectivorous plants, can capture and digest animal prey, absorb metabolites (nutrients) from killed prey, and utilize them for plant growth and development [1, 2]. Approximately 810 carnivorous plant species have been recognized, with several new species being described continuously and Triantha was the most recently described [1, 3]. Carnivory has evolved at least 13 times independently during the evolution of flowering plants with four origins in the monocot (three in Poales and one in Alismatales) and nine in eudicots (three in Caryophyllales, three in Lamiales, two in Ericales and one in Oxalidales), giving rise to 21 plant genera in 13 families of six orders [3,4,5,6,7,8]. Nearly 98% of carnivorous species belong to the four plant families Lentibulariaceae, Droseraceae, Nepenthaceae, and Sarraceniaceae, and the remaining nine families harbor only one or a few carnivorous representatives [5].
Convergently evolved modified leaves allowed the carnivorous lineages to expand their functional–anatomical realm to consume prey (Fig. 1). Pitfall (“pitcher”) traps (Fig. 1 C, D, H, J, K) could capture prey in pitcher-like modified leaves that contain a pool of digestive enzymes or bacteria. This trap type has evolved convergently at least six times in Sarraceniaceae (Sarracenia, Heliamphora, Darlingtonia), Cephalotaceae (Cephalotus), Nepenthaceae (Nepenthes), Eriocaulaceae (Paepalanthus), and twice in Bromeliaceae (Brocchinia and Catopsis, respectively) [8]. Adhesive (“flypaper”) traps (Fig. 1 B, E, F, I) use sticky mucilage on the leaf surface in Droseraceae (Drosera), Drosophyllaceae (Drosophyllum), Dioncophyllaceae (Triphyophyllum), Lentibulariaceae (**uicula), Roridulaceae (Roridula), Plantaginaceae (Philcoxia), and a recently described genus of Tofieldiaceae (Triantha) [3, 8, 9]. In contrast, the snap traps (Fig. 1 G, L) in Dionaeae and Aldrovanda (Droseraceae) utilize rapid leaf movements [9]. Lentibulariaceae exhibit two unique trap types: the eel (corkscrew) traps (Fig. 1 A1, A2) of Genlisea and the suction traps of Utricularia [10]. Eel traps form inward-pointing hairs to force prey to move toward the digestive organ; suction traps suck in prey by generating an internal vacuum in bladder-like leaves [11, 12].
Photos of representative carnivorous plants in this study. (A1) Genlisea filiformis, (A2) traps of Genlisea filiformis, (B) **uicula ehlersiae, (C) Darlingtonia californica, (D) Sarracenia alata, (E) Roridula gorgonias, (F) Drosera rotundifolia, (G) Dionaea muscipula, (H) Nepenthes mirabilis, (I) Drosophyllum lusitanicum, (J) Cephalotus follicularis, (K) Brocchinia hechtioides, (L) Aldrovanda vesiculosa
Carnivorous species sharing the same trap type may still vary greatly regarding the diversity of trap structures and nutrient utilization methods. For example, Triphyophyllum only produces carnivorous leaves for a short time before the peak of the rainy season [13]. Two species of Roridula with adhesive traps cannot secrete their own digestive enzymes, but absorb nitrogen from feces of symbiotically associated hemipterans that live on the plant-captured prey [14,15,16]. Similarly, a few members of Nepenthes acquire nitrogen from the feces and urine of mutualistic mammals that they attract [17,18,19].
Most carnivorous plants are terrestrial, with the exception of aquatic or amphibious Aldrovanda vesiculosa and ca. 60 Utricularia species [1]. Terrestrial carnivorous plants have high habitat specificity and grow mainly in open, infertile, and moist sites, where they can hardly absorb nutrients. They often display a low photosynthesis rate and slow growth rate compared with non-carnivorous herbs [9, 20]. In contrast, aquatic carnivorous plants mostly grow in shallow standing, oligo-mesotrophic and dystrophic waters. They exhibit much higher photosynthesis rates and growth rates than terrestrial carnivorous plants and similar rates to non-carnivorous aquatic plants [2, 20, 21]. Prey animals supplying nutrients, particularly nitrogen (N), can increase photosynthesis rate and, furthermore, may stimulate nutrient uptake from roots for growth rate increase [2, 9].
Carnivorous plants generally absorb inorganic ions and small organic molecules via selective carriers, whereas direct absorption of a mixture of organic substances, including proteins, via endocytosis was also found in some carnivorous groups [22, 23]. Although it is still debated whether or not the carnivores can directly uptake organic substances as partial substitutes for photosynthesis, recent studies have shown that amino acids absorbed from prey may serve as organic carbon sources for respiratory energy gain in Dionaea muscipula [24, 25], implying that carnivory possibly coincides with a partially heterotrophic or mixotrophic lifestyle in some lineages [9, 26].
Heterotrophic plants show some common characteristics in their plastome, including a small genome size, a reduced coding repertoire and noncoding region, genomic rearrangement, a high AT content, elevated substitution rate, etc. (reviewed in [27]). Distinctive photosynthesis rate and mixotrophic feeding strategy in carnivorous lineages imply that carnivorous plants may have distinct evolutionary pattern in their plastome. It was hypothesized that plastomes of carnivores might depart from the plastome stasis of most angiosperm lineages, with resemblance to the molecular-evolutionary trajectory of heterotrophic plants [26]. Corroborating this hypothesis are the observation of extraordinarily reconfigured plastomes in the Droseraceae, where multiple rearrangements, gene losses, and large expansions or contractions of the inverted repeats (IRs) occur in all the investigated species [28, 29]. For example, all ndh genes were lost from Droseraceae species, and some representatives also lack plastid-encoded genes like clpP, ycf1, ycf2, and some tRNAs. Lentibulariaceae exhibit a relatively conserved plastome structure, with the exception of massive ndh gene losses in all terrestrial, but not aquatic species [26, 30]. Additionally, altered proportions of repeat DNA, a significant plastome-wide increase of substitution rates and microstructural changes (indels) were observed in Lentibulariaceae plastomes [26]. In contrast, conserved plastomes with or without ndh genes losses characterize the plastome of Cephalotus follicularis [31] and Nepenthes × ventrata [29], respectively.
Changing from autotrophy to mixo- or heterotrophy is thought to have an impact on plastid genome evolution [2, 32]. Partial carnivorous nutrition may mitigate the selective pressure on plastome, resulting in a shift in substitution rate and gene loss in some lineages [26]. It is, however, unknown to date as to whether the available and taxonomically under-represented data, ranging from none to dramatic changes are associated with the varying extent of the implementation of the carnivorous syndrome in plants or due to other environmental factors. Besides, the focus on individual carnivorous species combined with the use of different analysis approaches could not track all aspects of potentially carnivorous-associated molecular-evolutionary paths. We aimed to fill this gap by broadly examining the plastomes of 28 carnivorous plants representing the majority of carnivorous families (9 out of 13) and their closely related non-carnivorous relatives across five angiosperm orders. Our phylogenomic comparative approach answers or discusses the following questions: 1) whether the nutritional benefit of carnivory impact the evolution of plastome, 2) whether the plastome evolutionary trajectories are convergent across in carnivorous plants, and 3) if the evolutionary patterns were different, what the underlying causes related to plastome variation in carnivorous plants are. Our results provide insight into the distinct evolutionary pattern of plastome across carnivorous lineages in angiosperms.
Results
Structural diversity of plastid genome in carnivorous plants
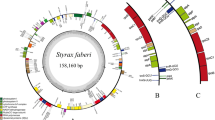
The plastomes of carnivorous plants exhibit a wide range of structural diversity range from highly conservation to significant variations in genome size, IR length, gene content, genomic arrangements, and repeat structure (Supporting Table S1, Table S3). For example, plastome sizes changed substantially in Droseraceae, ranging from 117,589 bp in Dionaea muscipula to 192,912 bp in Drosera rotundifolia. Genome sizes of other carnivorous species ranged from 139,725 bp (Utricularia reniformis) to 161,051 bp (Heliamphora minor) (Fig. 2, Supporting Table S1). Compared to their non-carnivorous relatives, genome sizes have reduced in all sampled species of Droseraceae except for the dramatic expansion in Drosera rotundifolia, and also in all terrestrial Lentibulariaceae species, Triphyophyllum peltatum, Drosophyllum lusitanicum, and Cephalotus follicularis. Plastome sizes vary slightly in Roridula gorgonias, Brocchinia hechtioides, two species of Nepenthes, and the aquatic Utricularia. The lengths of IRs in Droseraceae differ more than 18-fold, ranging from 2.8 kb in Dionaea muscipula to ~ 52.9 kb in Drosera rotundifolia. An expansion of the IR region was also observed in Sarraceniaceae species, and contraction of IR region was observed in both the carnivorous and non-carnivorous Dioncophyllaceae species. Other carnivorous plants maintained a conserved typical IR region (24,101–27,905 bp). The noncoding regions were extended in Sarraceniaceae species and contracted in Triphyophyllum peltatum (Fig. 2, Supporting Table S1).
Variations in the genome structure and IR lengths of carnivorous plants and their non-carnivorous relatives. The phylogenetic relationship is constructed using all the plastid protein coding genes with all samples, and the support value for each node was shown in Figure S1. Branches leading to Carnivorous lineages (blue text) are shown with thick lines with blue color, and the families that they belong to are listed in the upper left corner with the alphabet marked in each node. Grey rectangles represent IR regions, and red rectangles represent genome rearrangements (inversions) in carnivorous species compared to non-carnivorous relatives. Red lines in Droseraceae species represent dramatic rearrangements, and a detailed rearrangement picture showed in Figure S3
Drastic rearrangements of the plastid genome occurred in all sampled Droseraceae species (Fig. 2, Supporting Figure S3). Besides, a large inversion was observed in the plastomes of Utricularia amethystine, **uicula ehlersiae, Darlingtonia californica, and Triphyophyllum peltatum (Fig. 2, Supporting Figure S3, Supporting Table S1). The plastome structures of the remaining 22 carnivorous species were collinear with those of typical angiosperms, lacking any structural reconfigurations or gene relocations.
Carnivorous plants generally showed similar repeat content as non-carnivorous plants, yet Drosera rotundifolia had an extremely high number of repeats larger than 20 bp (1,089) (Supporting Figure S4, Table S1). Besides, Triphyophyllum peltatum (Dioncophyllaceae) showed more total repeats (340) than the sampled non-carnivorous relatives (82–205). The repeat content varied dramatically across species of Droseraceae (ranging from 72 to 1,089) and Lentibulariaceae (ranging from 44 to 215). The repeat contents of other carnivorous plants were similar to their non-carnivorous relatives or did not show consistent variation. Repeats of 50–100 bp and larger than 100 bp in length were rarely found in both the carnivorous and non-carnivorous plants, with minor departures in some species of Droseraceae and Triphyophyllum (Supporting Figure S4, Table S1).
The patterns of gene losses in plastid genomes
Compared to non-carnivorous relatives, gene losses or pseudogenization have occurred in all but four sampled carnivorous species (Brocchinia hechtioides, Triphyophyllum peltatum, Utricularia foliosa, and Utricularia amethystina) (Fig. 3). In general, ndh genes were lost in five out of nine carnivorous lineages and other genes including ccsA, clpP, infA, psbK, rpl23, rpl32, rps16, ycf1, ycf2, and some tRNA genes were only independently lost in few carnivorous species. In Droseraceae, all of the eleven ndh genes and clpP have been lost. Three more genes (trnA-UGC, trnV-UAC, and ycf1) were lost from the plastome of Drosera rotundifolia, and nine more genes (psbK, rpl23, rpl32, rps16, ycf1, ycf2, trnA-UGC, trnI-GAU, and trnV-UAC) are absent from Drosera erythrorhiza plastomes; Dionaea muscipula has also lost the trnV-UAC gene. In Lentibulariaceae, all eleven ndh genes were lost from the plastomes of all the sampled Genlisea species and Utricularia reniformis; in **uicula ehlersiae, the ndhA, C, D, E, F, G, I, and K genes were lost or pseudogenized. The ycf1 gene was not annotated in the published plastome of four species (G. margaretae, U. macrorhiza, U. gibba and P. ehlersiae), due to assumed sequencing technology-based errors [26]. In Sarraceniaceae, different numbers of ndh genes were lost in the three genera separately. The ndhA, D, E, F, G, I, and K genes were lost from the plastome of Heliamphora minor, the ndhA, B, C, D, F, G, H, I, J, and K genes were lost or pseudogenized in the plastome of Sarracenia alata, and ndh A, B, C, D, F, G, I, J, and K genes were lost in Darlingtonia califomica. In the plastome of Cephalotus follicularis (Cephalotaceae), all ndh genes and the infA gene were missing; the rpl32 gene represents a shared loss in Cephalotaceae and its relatives Brunelliaceae and Elaeocarpaceae. In the plastome of Drosophyllum lusitanicum (Drosophyllaceae), nine ndh genes (ndhA, B, C, D, F, G, H, I, K) were lost. The carnivorous plant in the other four families Roridulaceae, Nepenthaceae, Dioncophyllaceae, and Bromeliaceae encode all ndh genes in their plastomes. However, the clpP gene was missing from the plastome of both Roridula gorgonias (Roridulaceae) and its sampled close relatives of Actinidiaceae, and ccsA gene is a pseudogene in the plastome of two Nepenthes species (Nepenthaceae) (Fig. 3, Supporting Figure S5).
The extent of gene losses across carnivorous and non-carnivorous lineages. Branches leading to carnivorous lineages (blue text) are shown with thick lines in blue color. Commonly lost genes are list in the internal nodes; species-specific lost genes are listed at the tip nodes. The “φ” symbol before a gene name represents a pseudogene, and “?” symbol before a gene name represents an uncertain gene loss
Nucleotide substitution rate variation and its association with carnivory
Many carnivorous lineages shown distinct but not necessarily higher substitution rates compared to their non-carnivorous relatives. The overall significant elevation was only detected in the two largest carnivorous families, Lentibulariaceae and Droseraceae (Fig. 4; Supporting Figures S6, S7, and Table S4). A moderate acceleration of synonymous substitution rate was observed in many genes of Cephalotus follicularis, Roridula gorgonias, and Drosophyllum lusitanicum compared with their non-carnivorous relatives (Fig. 3, Supporting Figure S6, S7). In Triphyophyllum peltatum, approximately half of its plastid genes have increased substitution rates than non-carnivorous relatives and half of the genes evolve slower. Carnivorous Nepenthes and Sarraceniaceae species have more genes with lower substitution rates than their non-carnivorous relatives, but most ndh genes in plastome of Sarraceniaceae show increased dN and dS values (Supporting Table S4).
A TraitRateProp analysis showed that substitution rate differences were associated with carnivory in many lineages (Supporting Table S5). A large number of plastid genes of Roridulaceae (63 out of 78 genes), Lentibulariaceae (55 out of 79 genes), Droseraceae (54 out of 67 genes), Nepenthaceae (46 out of 78 genes), Sarraceniaceae (46 out of 72 genes), and Drosophyllaceae (46 out of 70 genes) showed significant lifestyle-associated substitution rates change. Moderate numbers of genes with significant substitution rates change characterize Cephalotaceae (34 out of 65 genes), Bromeliaceae (32 out of 79 genes), and Dioncophyllaceae (25 out of 79 genes) plastomes. Significant differences in substitution rates occurred in both photosynthesis and housekee** genes across all the carnivorous families alike. Substitution rates of ndhA, D, F and H genes evolved significantly different in all ndh genes-retained carnivorous lineages, and substitution rates of many other genes including accD, atpA, atpB, ccsA, matK, petA, psaA, psaB, psbB, psbC, rbcL, rpoA, rpoB, rpoC1, rpoC2, rps3, ycf1 and ycf2 genes differed significantly in all carnivorous compared with non-carnivorous relatives (Supporting Table S5).
Changes in the selection of plastid genes
Both photosynthesis-related genes and housekee** genes showed significant shifts in selection in some carnivorous families relative to their non-carnivorous relatives, though the patterns of selectional shifts differed across lineages. Separately, nine genes in the carnivorous lineage of Lentibulariaceae (accD, atpE, clpP, petL, psbK, rbcL, rpoC1, ycf1, ycf2), seven in Nepenthaceae (atpA, cemA, clpP, psbC, rpl20, rpoB, ycf1), six in Drosophyllaceae (clpP, psbJ, rbcL, rpl20, rpoB, ycf2) and Sarraceniaceae (accD, atpA, petL, rpl22, ycf1, ycf2), five in Droseraceae (atpA, cemA, rpl14, rpoC1, ycf1) and Roridulariaceae (psbB, rpoB, rpoC1, rps11, ycf1), four in Cephalotaceae (clpP, petD, psbJ, rbcL), and two in Bromeliaceae (rps2, cemA) exhibited significantly relaxed selective constraints associated with carnivory; no such shifts were detected in Dioncophyllaceae (Table 1, Supporting Table S6). The ycf1 genes evolved under relaxed selective constraint in five out of nine carnivorous lineages, while other genes evolved under relaxed selection in a few carnivorous lineages. In contrast, many genes appear to experience intensified selection, including four (ndhC, E, H, J) out of five remaining intact ndh genes (ndhB, C, E, H, J) in Sarraceniaceae.
Discussion
Plastome structure variations in carnivorous plants
The carnivory in angiosperms only affect the plastome structure in a few lineages and showed different patterns. Most of our sampled carnivorous lineages harbor a conserved plastome structure. Except for the dramatic rearrangements in Droseraceae species, structural reconfigurations are generally minor and species-specific in Utricularia amethystine, **uicula ehlersiae, Darlingtonia californica, and Triphyophyllum peltatum (Fig. 2). Elevated amounts of plastid repeat of longer than 12 bp but not longer than 50 bp were previously described in carnivorous Lentibulariaceae [26]. Considering repeats of 20 bp or longer, there are no universal differences between carnivorous and their closely non-carnivorous relatives in this study (Figure S4). Carnivorous species with inversions tend to possess more repeats larger than 50 bp or 100 bp, which may reveal a correlation between the accumulation of large repeats and genome rearrangement reported before [33, 34].
The number of genes, GC content, extension/contraction of IR region, and noncoding regions can greatly affect genome length and stability [32, 35, 36]. In carnivorous species, the variation in plastome length can be attributed to different factors. Gene content underlies plastome size variation in many lineages, including Lentibulariaceae, most Droseraceae species, as well as in Drosophyllum lusitanicum and Cephalotus follicularis. However, the extension of both IRs and noncoding regions of Sarraceniaceae species and Drosera rotundifolia contribute considerably more to plastome size inflation. In contrast, Triphyophyllum peltatum, which retains a complete set of plastid genes, exhibits a shorter plastome than its non-carnivorous relatives, mainly due to the contraction of IRs and noncoding regions (Supporting Table S1). Condensation of non-coding regions and deletion of non-essential DNA was also previously described in Lentibulariaceae plastomes, where the overall number of deletions exceeded that of insertions [26], which is also seen in parasitic plants [37].
Plastid gene loss in carnivorous plants
The loss of plastid genes occurred independently in many carnivorous lineages. In this study, we detected gene losses or pseudogenization in all but four sampled carnivorous species (Brocchinia hechtioides, Triphyophyllum peltatum, Utricularia foliosa, and Utricularia amethystina) (Fig. 3). The loss of ndh genes is the most widespread, which were independently occurred in five out of nine carnivorous lineages. The loss of other genes was generally rare and independent. Only a few carnivorous species retained all ndh genes, including four aquatic representatives of Utricularia, the terrestrial species Brocchinia hechtioides, Roridula gorgonias, Triphyophyllum peltatum, and two Nepenthes species. Plastid ndh genes encode components of the thylakoid NAD(P)H dehydrogenase complex, which adjusts the redox level of cyclic photosynthetic electron transporters [38]. The genes were identified to be essential for plants under stress conditions [39, 40], but appears to be dispensable under favorable growth conditions, and holds limited biological significance in modern plants [41, 42]. The absence of plastid ndh genes were noted across photoautotrophic seed plants both in gymnosperms and angiosperms [33, 43,44,45,46,47], and it occurred prevalently in plants that no longer entirely rely on photosynthesis for energy and nutrients, such as parasitic and mycoheterotrophic groups. This absence represents the initial stage of plastome degradation in heterotrophic plants [27, 32, 37, 47,48,49,50,51]. Multiple independent losses of the ndh complex reveal that carnivory in plants may mitigate environmental stress or that prey-derived nutrients in some carnivorous lineages alleviate the selective pressure for the ndh complex in nutrient poor environment. The patterns and extent of ndh gene deletions may reflect the evolutionary trajectories of carnivory in angiosperms.
Except for ndh genes, we also found a few other photosynthesis related genes to be lost or pseudogenized, including ccsA in Nepenthes species, and psbK, rpl23, rpl32, rps16 in Drosera erythrorhiza. In contrast, all other photosynthesis-related genes are maintained in all other carnivorous species. The preservation of the majority of photosynthesis genes corresponds to the relative normal photosynthesis capacity of carnivorous groups, but corroborates the dispensable role of the plastid ndh complex in these plants. Besides this, the clpP gene, known to be indispensable for cell survival [52], was lost in Droseraceae and Roridula-Actinidiaceae. This gene was also reported to be lost in other Actinidiaceae species and it might be a synapomorphy for the sister groups [53]. Loss or pseudogenization of clpP was also reported in some heterotrophic species like mycoheterotrophic Ericaceae [48] and parasitic Hydnora [54], characterizing a late stage of plastome degradation [27]. The ycf1 gene was identified to have an essential function for chloroplasts [55]. Nonetheless, it was reported to be lost in grasses, some parasitic species, Vaccinium, and Erodium [56], and, here, in some carnivorous plants. This gene was independently lost in at least two Drosera species (D. rotundifolia and D. erythrorhiza), and it remains to be clarified if the gene is functional in Lentibulariaceae [26]. The ccsA gene is required for heme attachment to chloroplast c-type cytochromes [79] and BLAST + [80] based on reference database plugin the toolkit. Subsequently, the potential plastome-derived reads were de novo-assembled using SPAdes version 3.6.2 [81]. The analysis was performed at the iFlora High-Performance Computing Center of Germplasm Bank of Wild Species (iFlora HPC Center of GBOWS, KIB, CAS). Both the newly obtained and published plastome sequences were annotated with PGA [82], with manual correction and modification in Geneious v9.0.2 (Biomatters Limited). Pseudogenes were defined based on the loss of parts in their sequences or by the presence of internal stop codons, which would be caused by nucleotide mutation, frameshift mutation, premature stop codon, etc. [37]. The collinearity of plastomes between carnivorous plants and their close relatives was assessed using the Mauve v. 2.3.1 [83, 84] plugin in Geneious. The genome size, length of the large and small single-copy regions (LSC, SSC) and inverted repeat regions (IRs), gene numbers, and GC content of each species were summarized using Geneious. Direct and palindromic long repeats were detected using REPuter (https://bibiserv.cebitec.uni-bielefeld.de/reputer; [85]), with a minimal repeat size of 20 bp and a Hamming distance of 1.
Substitution rate and selection pressure analyses
The protein coding genes were extracted using PhyloSuite v1.2.2 [86]. Each gene was aligned using MAFFT v7.22 [87] plugin in PhyloSuite with G-INS-I algorithm and “translation align” option selected. All the genes were concatenated in PhyloSuite to generate a supermatrix.
To test for changes in substitution rates and selection pressure, a phylogenetic tree including all taxa as well as nine subtrees of carnivorous and non-carnivorous pairs were constructed using RAxML v8.1.11 [88, 89]. Tree reconstruction was carried out with the GTR + GAMMA model based on a concatenated supermatrix of all plastid protein coding genes with all taxa and nine subsets (A total tree and nine subtrees of carnivorous and non-carnivorous pair clade shown in Supporting Figure S1) separately.
The nonsynonymous rates (dN) and synonymous rates (dS) were estimated for each gene using PAML v. 4.7 [90] in codeml mode, with branch models (model = 1, free-ratios model) based on each gene matrix and the total tree. To compare substitution rate in the carnivorous and non-carnivorous lineages, the tip-to-root branch length for each species was calculated using software Newick Utilities [91].
The TraitRateProp method [92, 93] was used to test for associations between carnivory and substitution rates for each protein-coding gene of our nine carnivorous and non-carnivorous pairs set. A time tree including all samples was estimated using penalized likelihood method using treePL software [94] with ten secondary calibrations according to previous publications (Supporting Table S2). Nine dated subtrees were extracted from the whole time-dated tree (Supporting Figure S2) for the later analyses. Carnivory was coded as a binary trait (1 = carnivorous plant; 0 = non-carnivorous plant). The method was used to test the hypothesis of a trait-rate association against a null model of no association via stochastic map**.
The RELAX model in HyPhy v 2.5.36 [95, 96] was used to test the hypothesis of relaxed and intensified selective constrains in carnivorous lineages versus their non-carnivorous relatives. The carnivorous lineage was set as “test” branches, and their most closely related non-carnivorous lineage were set as “reference” branches for each carnivorous and non-carnivorous subtree (Supporting Figure S1). The method compared two models, with a null model of same ω distribution on test and reference branches against an alternative model in which branches are allowed to have different ω distribution in a likelihood ratio test (LRT). The analyses were run via command line on a local server, and resulting ‘.json’ files were parsed with a custom python script.
Availability of data and materials
All DNA sequences have been deposited in the NCBI GenBank database (accession numbers in Table S1).
References
Ellison AM, Adamec L. In: Carnivorous plants: physiology, ecology, and evolution. Oxford, UK: Oxford University Press; 2018.
Adamec L, Matusikova I, Pavlovic A. Recent ecophysiological, biochemical and evolutional insights into plant carnivory. Ann Bot. 2021;128(3):241–59.
Lin Q, Ane C, Givnish TJ, Graham SW. A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap. Proc Natl Acad Sci U S A. 2021;118(33): e2022724118.
Müller K, Borsch T, Legendre L, Porembski S, Theisen I, Barthlott W. Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biol. 2004;6(4):477–90.
Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants—Darwin’s ‘most wonderful plants in the world.’ J Exp Bot. 2009;60(1):19–42.
Ellison AM, Butler ED, Hicks EJ, Naczi RF, Calie PJ, Bell CD, Davis CC. Phylogeny and biogeography of the carnivorous plant family Sarraceniaceae. PLoS One. 2012;7(6): e39291.
Yao G, ** JJ, Li HT, Yang JB, Mandala VS, Croley M, Mostow R, Douglas NA, Chase MW, Christenhusz MJ. Plastid phylogenomic insights into the evolution of Caryophyllales. Mol Phylogen Evol. 2019;134:74–86.
Givnish TJ. New evidence on the origin of carnivorous plants. Proc Natl Acad Sci U S A. 2015;112(1):10–1.
Pavlovic A, Saganova M. A novel insight into the cost-benefit model for the evolution of botanical carnivory. Ann Bot. 2015;115(7):1075–92.
Płachno BJ, Muravnik LE. Functional anatomy of carnivorous traps. In: Ellison AM, AL, editors. Carnivorous plants : physiology, ecology, and evolution. Oxford, UK: Oxford University Press; 2018. p. 167–79.
Fleischmann A, Schaferhoff B, Heubl G, Rivadavia F, Barthlott W, Muller KF. Phylogenetics and character evolution in the carnivorous plant genus Genlisea A. St.-Hil. (Lentibulariaceae). Mol Phylogenet Evol. 2010; 56(2):768–783.
Reifenrath K, Theisen I, Schnitzler J, Porembski S, Barthlott W. Trap architecture in carnivorous Utricularia (Lentibulariaceae). Flora Morphol Distrib Funct Ecol Plants. 2006;201(8):597–605.
Green S, Green TL, Heslop-Harrison Y. Seasonal heterophylly and leaf gland features in Triphyophyllum (Dioncophyllaceae), a new carnivorous plant genus. Bot J Linn Soc. 1979;78(2):99–116.
Ellis AG, Midgley JJ. A new plant-animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia. 1996;106(4):478–81.
Anderson B, Midgley JJ. Digestive mutualism, an alternate pathway in plant carnivory. Oikos. 2003;102(1):221–4.
Anderson B, Midgley JJ. Density-dependent outcomes in a digestive mutualism between carnivorous Roridula plants and their associated hemipterans. Oecologia. 2007;152(1):115–20.
Bazile V, Moran JA, Le Moguedec G, Marshall DJ, Gaume L. A carnivorous plant fed by its ant symbiont: a unique multi-faceted nutritional mutualism. PLoS One. 2012;7(5): e36179.
Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biol Lett. 2009;5(5):632–5.
Grafe TU, Schoner CR, Kerth G, Junaidi A, Schoner MG. A novel resource-service mutualism between bats and pitcher plants. Biol Lett. 2011;7(3):436–9.
Ellison AM, Adamec L. Ecophysiological traits of terrestrial and aquatic carnivorous plants: are the costs and benefits the same? Oikos. 2011;120(11):1721–31.
Adamec L. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquat Bot. 2008;89(1):66–70.
Adlassnig W, Koller-Peroutka M, Bauer S, Koshkin E, Lendl T, Lichtscheidl IK. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012;71(2):303–13.
Koller-Peroutka M, Krammer S, Pavlik A, Edlinger M, Lang I, Adlassnig W. Endocytosis and digestion in carnivorous pitcher plants of the family Sarraceniaceae. Plants. 2019;8(10):367.
Fasbender L, Maurer D, Kreuzwieser J, Kreuzer I, Schulze WX, Kruse J, Becker D, Alfarraj S, Hedrich R, Werner C, et al. The carnivorous Venus flytrap uses prey-derived amino acid carbon to fuel respiration. New Phytol. 2017;214(2):597–606.
Kruse J, Gao P, Eibelmeier M, Alfarraj S, Rennenberg H. Dynamics of amino acid redistribution in the carnivorous Venus flytrap (Dionaea muscipula) after digestion of (13) C/(15) N-labelled prey. Plant Biol. 2017;19(6):886–95.
Wicke SK, Schaeferhoff B, dePamphilis CW, Mueller KF. Disproportional plastome-wide increase of substitution rates and relaxed purifying selection in genes of carnivorous Lentibulariaceae. Mol Biol Evol. 2014;31(3):529–45.
Wicke S, Naumann J. Molecular evolution of plastid genomes in parasitic flowering plants. In: Shu-Miaw Chaw RKJ, editor. Plastid Genome Evolution. London, UK: Academic Press; 2018. p. 315–47.
Nevill PG, Howell KA, Cross AT, Williams AV, Zhong X, Tonti-Filippini J, Boykin LM, Dixon KW, Small I. Plastome-wide rearrangements and gene losses in carnivorous Droseraceae. Genome Biol Evol. 2019;11(2):472–85.
Gruzdev EV, Kadnikov VV, Beletsky AV, Kochieva EZ, Mardanov AV, Skryabin KG, Ravin NV. Plastid genomes of carnivorous plants Drosera rotundifolia and Nepenthes x ventrata reveal evolutionary patterns resembling those observed in parasitic plants. Int J Mol Sci. 2019;20(17):4107.
Silva SR, Diaz YCA, Penha HA, Pinheiro DG, Fernandes CC, Miranda VFO, Michael TP, Varani AM. The Chloroplast genome of Utricularia reniformis sheds light on the evolution of the ndh gene complex of terrestrial carnivorous plants from the Lentibulariaceae family. PLoS One. 2016;11(10): e0165176.
Cao M, Li Z, Dai X, Wu X, Li Y, Wu S. The complete plastid genome of carnivorous pitcher plant Cephalotus follicularis. Mitochondrial DNA Part B. 2019;4(1):2025–7.
Wicke S, Muller KF, dePamphilis CW, Quandt D, Bellot S, Schneeweiss GM. Mechanistic model of evolutionary rate variation en route to a nonphotosynthetic lifestyle in plants. Proc Natl Acad Sci U S A. 2016;113(32):9045–50.
Weng ML, Blazier JC, Govindu M, Jansen RK. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol Biol Evol. 2014;31(3):645–59.
Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76(3):273–97.
Zhu A, Guo W, Gupta S, Fan W, Mower JP. Evolutionary dynamics of the plastid inverted repeat: the effects of expansion, contraction, and loss on substitution rates. New Phytol. 2015;209(4):1747–56.
Roschenbleck J, Wicke S, Weinl S, Kudla J, Muller KF. Genus-wide screening reveals four distinct types of structural plastid genome organization in Pelargonium (Geraniaceae). Genome Biol Evol. 2017;9(1):64–76.
Cusimano N, Wicke S. Massive intracellular gene transfer during plastid genome reduction in nongreen Orobanchaceae. New Phytol. 2016;210(2):680–93.
Martin M, Sabater B. Plastid ndh genes in plant evolution. Plant Physiol Biochem. 2010;48(8):636–45.
Endo T, Shikanai T, Takabayashi A, Asada K, Sato F. The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett. 1999;457(1):5–8.
Ma M, Liu Y, Bai C, Yong JWH. The significance of chloroplast NAD(P)H dehydrogenase complex and its dependent cyclic electron transport in photosynthesis. Front Plant Sci. 2021;12: 661863.
Lin CS, Chen JJW, Chiu CC, Hsiao HCW, Yang CJ, ** XH, Leebens-Mack J, de Pamphilis CW, Huang YT, Yang LH, et al. Concomitant loss of NDH complex-related genes within chloroplast and nuclear genomes in some orchids. Plant J. 2017;90(5):994–1006.
Ruhlman TA, Chang WJ, Chen JJ, Huang YT, Chan MT, Zhang J, Liao DC, Blazier JC, ** X, Shih MC, et al. NDH expression marks major transitions in plant evolution and reveals coordinate intracellular gene loss. BMC Plant Biol. 2015;15:100.
Sun Y, Moore MJ, Lin N, Adelalu KF, Meng A, Jian S, et al. Complete plastome sequencing of both living species of Circaeasteraceae (Ranunculales) reveals unusual rearrangements and the loss of the ndh gene family. BMC Genomics. 2017;18(1):1–10.
Deng N, Hou C, Liu C, Li M, Bartish I, Tian Y, Chen W, Du C, Jiang Z, Shi S. Significance of photosynthetic characters in the evolution of asian Gnetum (Gnetales). Front Plant Sci. 2019;10:39.
Chumley TW, Palmer JD, Mower JP, Fourcade HM, Calie PJ, Boore JL, Jansen RK. The complete chloroplast genome sequence of Pelargonium x hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol. 2006;23(11):2175–90.
Wu CS, Lin CP, Hsu CY, Wang RJ, Chaw SM. Comparative chloroplast genomes of pinaceae: insights into the mechanism of diversified genomic organizations. Genome Biol Evol. 2011;3:309–19.
Wicke S, Muller KF, de Pamphilis CW, Quandt D, Wickett NJ, Zhang Y, Renner SS, Schneeweiss GM. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell. 2013;25(10):3711–25.
Braukmann TWA, Broe MB, Stefanović S, Freudenstein JV. On the brink: the highly reduced plastomes of nonphotosynthetic Ericaceae. New Phytol. 2017;216(1):254–66.
Logacheva MD, Schelkunov MI, Shtratnikova VY, Matveeva MV, Penin AA. Comparative analysis of plastid genomes of non-photosynthetic Ericaceae and their photosynthetic relatives. Sci Rep. 2016;6:30042.
Wu CS, Wang TJ, Wu CW, Wang YN, Chaw SM. Plastome evolution in the sole hemiparasitic genus laurel dodder (Cassytha) and insights into the plastid phylogenomics of Lauraceae. Genome Biol Evol. 2017;9(10):2604–14.
Bungard RA. Photosynthetic evolution in parasitic plants: insight from the chloroplast genome. BioEssays. 2004;26(3):235–47.
Shikanai T, Shimizu K, Ueda K, Nishimura Y, Kuroiwa T, Hashimoto T. The chloroplast clpP gene, encoding a proteolytic subunit of ATP-dependent protease, is indispensable for chloroplast development in tobacco. Plant Cell Physiol. 2001;42(3):264–73.
Wang WC, Chen SY, Zhang XZ. Chloroplast genome evolution in Actinidiaceae: clpP loss, heterogenous divergence and phylogenomic practice. PLoS One. 2016;11(9):17.
Naumann J, Der JP, Wafula EK, Jones SS, Wagner ST, Honaas LA, Ralph PE, Bolin JF, Maass E, Neinhuis C, et al. Detecting and characterizing the highly divergent plastid genome of the nonphotosynthetic parasitic plant Hydnora visseri (Hydnoraceae). Genome Biol Evol. 2016;8(2):345–63.
Drescher A, Ruf S, Calsa T Jr, Carrer H, Bock R. The two largest chloroplast genome-encoded open reading frames of higher plants are essential genes. Plant J. 2000;22(2):97–104.
de Vries J, Sousa FL, Bolter B, Soll J, Gould SB. YCF1: A green TIC? Plant Cell. 2015;27(7):1827–33.
**e Z, Merchant S. The plastid-encoded ccsA gene is required for heme attachment to chloroplast c-type cytochromes. J Biol Chem. 1996;271(9):4632–9.
Park S, Jansen RK, Park S. Complete plastome sequence of Thalictrum coreanum (Ranunculaceae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol. 2015;15:40.
Millen RS, Olmstead RG, Adams KL, Palmer JD, Lao NT, Heggie L, Kavanagh TA, Hibberd JM, Gray JC, Morden CW, et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 2001;13(3):645–58.
Ueda M, Fujimoto M, Arimura S, Murata J, Tsutsumi N, Kadowaki K. Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene. 2007;402(1–2):51–6.
Bromham L, Cowman PF, Lanfear R. Parasitic plants have increased rates of molecular evolution across all three genomes. BMC Evol Biol. 2013;13(1):126.
Sloan DB. Using plants to elucidate the mechanisms of cytonuclear co-evolution. New Phytol. 2015;205(3):1040–6.
Allen JF. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc Natl Acad Sci U S A. 2015;112(33):10231–8.
Weng ML, Ruhlman TA, Jansen RK. Plastid-nuclear interaction and accelerated coevolution in plastid ribosomal genes in Geraniaceae. Genome Biol Evol. 2016;8(6):1824–38.
Forsythe ES, Williams AM, Sloan DB. Genome-wide signatures of plastid-nuclear coevolution point to repeated perturbations of plastid proteostasis systems across angiosperms. Plant Cell. 2021;33(4):980–97.
Zhang J, Ruhlman TA, Sabir JS, Blazier JC, Weng ML, Park S, Jansen RK. Coevolution between nuclear-encoded DNA replication, recombination, and repair genes and plastid genome complexity. Genome Biol Evol. 2016;8(3):622–34.
Jobson RW, Albert VA. Molecular rates parallel diversification contrasts between carnivorous plant sister lineages. Cladistics. 2002;18(2):127–36.
Ibarra-Laclette E, Albert VA, Perez-Torres CA, Zamudio-Hernandez F, Ortega-Estrada Mde J, Herrera-Estrella A, Herrera-Estrella L. Transcriptomics and molecular evolutionary rate analysis of the bladderwort (Utricularia), a carnivorous plant with a minimal genome. BMC Plant Biol. 2011;11:101.
Barrett CF, Sinn BT, Kennedy AH. Unprecedented parallel photosynthetic losses in a heterotrophic Orchid genus. Mol Biol Evol. 2019;36(9):1884–901.
Suetsugu K, Yamato M, Miura C, Yamaguchi K, Takahashi K, Ida Y, Shigenobu S, Kaminaka H. Comparison of green and albino individuals of the partially mycoheterotrophic orchid Epipactis helleborine on molecular identities of mycorrhizal fungi, nutritional modes and gene expression in mycorrhizal roots. Mol Ecol. 2017;26(6):1652–69.
Gaut B, Yang L, Takuno S, Eguiarte LE. The patterns and causes of variation in plant nucleotide substitution rates. Annu Rev Ecol Evol Syst. 2011;42(1):245–66.
Płachno BJ, Adamec L, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 2006;8(6):813–20.
Anderson B, Midgley J. It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia. 2002;132(3):369–73.
Porembski S, Theisen I, Barthlott W. Biomass allocation patterns in terrestrial, epiphytic and aquatic species of Utricularia (Lentibulariaceae). Flora Morphol Distrib Funct Ecol Plants. 2006;201(6):477–82.
Brewer JS. Effects of competition, litter, and disturbance on an annual carnivorous plant (Utricularia juncea). Plant Ecol. 1999;140(2):159–65.
Kok PJR, Ratz S, Marco T, Aubret F, Means DB. Out of taxonomic limbo: a name for the species of Tepuihyla (Anura: Hylidae) from the Chimantá Massif, Pantepui region, northern South America. Salamandra. 2015;51:283–314.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–5.
** JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21(1):241.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):421.
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. Spades: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77.
Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50.
Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–403.
Darling AE, Mau B, Perna NT. ProgressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5(6): e11147.
Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29(22):4633–42.
Zhang D, Gao F, Jakovlic I, Zou H, Zhang J, Li WX, Wang GT. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2020;20(1):348–55.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–90.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3.
Yang Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–91.
Junier T, Zdobnov EM. The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics. 2010;26(13):1669–70.
Levy Karin E, Ashkenazy H, Wicke S, Pupko T, Mayrose I. TraitRateProp: a web server for the detection of trait-dependent evolutionary rate shifts in sequence sites. Nucleic Acids Res. 2017;45(W1):W260–4.
Levy Karin E, Wicke S, Pupko T, Mayrose I. An integrated model of phenotypic trait changes and site-specific sequence evolution. Syst Biol. 2017;66(6):917–33.
Smith SA, O’Meara BC. treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics. 2012;28(20):2689–90.
Wertheim JO, Murrell B, Smith MD, Kosakovsky Pond SL, Scheffler K. RELAX: detecting relaxed selection in a phylogenetic framework. Mol Biol Evol. 2015;32(3):820–32.
Pond SL, Frost SD, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21(5):676–9.
Acknowledgements
We are grateful to **g Yang, Juan Yang, and other supporting staff of the Germplasm Bank of Wild Species of Kunming Institute of Botany, the Chinese Academy of Sciences (CAS) and Dr. Jianfei Ye from the CAS Institute of Botany for hel** with sample collection and/or laboratory work; Drs. **yong Hu, Tingshuang Yi, and Weishu Fan for discussion of the data analyses; Wang **, Lian Tao, Zhiqiong Mo and Wei Zheng from CAS Kunming Institute of Botany for providing pictures of carnivorous species shown in Fig. 1. Molecular experiments and data analysis were performed at the Laboratory of Molecular Biology and iFlora High Performance Computing Center of Germplasm Bank of Wild Species respectively.
Funding
This study was supported by the National Natural Science Foundation of China (32000173), Special Research Assistant Funding Project of the Chinese Academy of Sciences (E0295111Q1), the Large-scale Scientific Facilities of the Chinese Academy of Sciences (2017-LSFGBOWS-02), the Key Basic Research program of Yunnan Province, China (202101BC070003) and the Postdoctoral Directional Training Foundation of Yunnan Province (E132711261).
Author information
Authors and Affiliations
Contributions
C.N.F. and L.M.G. designed the research. C.N.F. performed experiments and analyzed data. C.N.F., L.M.G., S.W., A.D.Z. and D.Z.L. interpreted the results and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the material collections in the study obtained the permission and followed the relevant institutional, national, and international guidelines and legislation. No specific permissions or licenses were needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The (A) total tree and (B) subtrees of nine pairs of carnivorous and non-carnivorous clades. The phylogenetic relationship is constructed using all the plastid protein coding genes. The support value for each node was shown in the total tree. Branches leading to carnivorous lineages (blue text) are shown with thick lines with blue color, and the closest non-carnivorous lineages in subtrees are shown with thick lines with orange color. Figure S2. Divergence time estimation of total tree with all samples. Branches leading to carnivorous lineages (blue text) are shown with thick lines with blue color. Figure S3. Mauve plot showing inversions in (A) Droseraceae, (B) Utricularia amethystine, (C) **uicula ehlersiae, (D) Darlingtonia califonica, and (E) Triphyophyllum peltatum compared to their non-carnivorous relatives. The blocks with the same color represent the genome region with similar nucleotide sequence and the blocks with same color but opposite orientation represent the genome region with inversion.Figure S4. Repeats content in carnivorous and non-carnivorous lineages. (A) The histogram shows the repeats content variation across carnivorous lineages and their non-carnivorous relatives. (B) Boxplot shows the difference in repeats content between carnivorous and non-carnivorous species. Figure S5. Gene content for each species. The black square means the gene is present in the species, the grey square means the gene is pseudogenized in the species, and the white square means the gene is absent from the species. Figure S6. The boxplot illustrates the difference in dN values between carnivorous and non-carnivorous species for each gene group of each carnivorous and non-carnivorous pair. The PS represents other photosynthesis genes, and HK represents other housekee** genes. The “*” symbol represents P < 0.05, “**” represents P < 0.01, “***” represents P < 0.001, and “****” represents P < 0.0001. Figure S7. The boxplot illustrates the difference in dS values between carnivorous and non-carnivorous species for each gene groups of each carnivorous and non-carnivorous pair. The PS represents other photosynthesis genes, and HK represents other housekee** genes. The “*” symbol represents P < 0.05, “**” represents P < 0.01, “***” represents P < 0.001, and “****” represents P < 0.0001.
Additional file 2:
Table S1. Information of sampled species and plastome characters of carnivorous speceis and their closely related non-carnivorous species. Table S2. The calibration nodes used in divergence time analyses. Table S3. The gene content of sampled species. Table S4. The nonsynonymous and synonymous substitution rates of sampled species.Table S5. Substitution rate change test for each protein-coding gene of nine carnivorous and non-carnivorous pairs.Table S6. Results of RELAX (HyPhy) analyses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, CN., Wicke, S., Zhu, AD. et al. Distinctive plastome evolution in carnivorous angiosperms. BMC Plant Biol 23, 660 (2023). https://doi.org/10.1186/s12870-023-04682-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04682-1