Abstract
Background
Nasopharyngeal carcinoma (NPC) is well-known for its highly metastatic characteristics, but little is known of its molecular mechanisms. New biomarkers that predict clinical outcome, in particular the ability of the primary tumor to develop metastatic tumors are urgently needed. The aim of this study is to investigate the role of FLJ10540 in human NPC development.
Methods
A bioinformatics approach was used to explore the potentially important regulatory genes involved in the growth/metastasis control of NPC. FLJ10540 was chosen for this study. Two co-expression strategies from NPC microarray were employed to identify the relationship between FLJ10540 and osteopontin. Quantitative-RT-PCR, immunoblotting, and immunohistochemistry analysis were used to investigate the mRNA and protein expression profiles of FLJ10540 and osteopontin in the normal and NPC tissues to confirm microarray results. TW01 and Hone1 NPC cells with overexpression FLJ10540 or siRNA to repress endogenous FLJ10540 were generated by stable transfection to further elucidate the molecular mechanisms of FLJ10540-elicited cell growth and metastasis under osteopontin stimulation.
Results
We found that osteopontin expression exhibited a positive correlation with FLJ10540 in NPC microarray. We also demonstrated comprehensively that FLJ10540 and osteopontin were not only overexpressed in NPC specimens, but also significantly correlated with advanced tumor and lymph node-metastasis stages, and had a poor 5-year survival rate, respectively. Stimulation of NPC parental cells with osteopontin results in an increase in FLJ10540 mRNA and protein expressions. Functionally, FLJ10540 transfectant alone, or stimulated with osteopontin, exhibited fast growth and increased metastasis as compared to vehicle control with or without osteopontin stimulation. Conversely, knockdown of FLJ10540 by siRNA results in the suppression of NPC cell growth and motility. Treatment with anti-CD44 antibodies in NPC parental cells not only resulted in a decrease of FLJ10540 protein, but also affected the abilities of FLJ10540-elicited cell growth and motility in osteopontin stimulated-NPC cells.
Conclusions
These findings suggest that FLJ10540 may be critical regulator of disease progression in NPC, and the underlying mechanism may involve in the osteopontin/CD44 pathway.
Similar content being viewed by others
Background
Nasopharyngeal carcinoma (NPC), the most common head and neck cancer, is a disease in which malignant cells form in the epithelium of the nasopharyngeal cavity. There is a high incidence of NPC in southern China and Southeast Asia, where the annual incidence is more than 20–30 cases per 100,000 people [Full size image
Using the concept of syn-expression to uncover osteopontin, as the upstream regulator of FLJ10540 in NPC specimens
Using the concept of syn-expression, genes belonging to the same pathway often show the same regulatory profiles under a variety of biological situations [30]. Hence, we employed the same publicly accessible microarray dataset, as described earlier, to gain insight into the functional concordance of co-expressed genes. To test this hypothesis, we first examined whether the mRNA expression profile of FLJ10540 correlated with any ligand in the publicly accessible microarray database of NPC. Of these co-expressed genes, the top candidate was osteopontin, which was highly positively correlated with the mRNA expression level of FLJ10540 in NPC cancer patients (r=0.72 p<0.001). This raises the possibility that these two molecules are functionally linked or in the same pathway.
The same panels of 12 NPC-tumor and five normal tissues were also applied to examine the mRNA expression level of osteopontin by Q-RT-PCR. As shown in Figure 1B, the mRNA expression levels of osteopontin were increased in the tumor samples as compared to those in normal tissue samples. The expressions of FLJ10540 and osteopontin in another batch of freshly frozen tumor and normal samples were further verified by western blotting. Our data indicated that the western blot results were consistent with the observed mRNA expressions of FLJ10540 and osteopontin in NPC specimens (Figure 1C). Taken together, these results suggest that there is a significant positively correlation between FLJ10540 and osteopontin in human NPC.
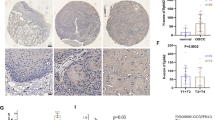
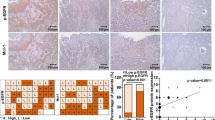
To investigate the clinical implications of FLJ10540 and osteopontin in NPC, immunohistochemical analysis was performed to determine the expressions and distributions of FLJ10540 and osteopontin in 63 human NPC samples and 10 normal tissues. The representative results of FLJ10540 and osteopontin immunostaining in NPC are shown in Figure 2A. First, FLJ10540 and osteopontin revealed very weak but detectable staining in the epithelium (Figure 2A, a and f). In the tumor samples, FLJ10540 and osteopontin expressions were positively correlated with tumor stage and node stage of the tumor cells (Figure 2A, b-e and g-j). However, strong stainings were not observed in tumor tissues when anti-FLJ10540 and anti-osteopontin antibodies were pre-incubated with protein corresponding to a portion of FLJ10540 and osteopontin, respectively (data not shown). Third, FLJ10540 and osteopontin were largely localized in the cytoplasm of the tumor sample cells as well as in normal tissues. To further confirm our immunohistochemisty results, anti-FLJ10540 and anti-osteopontin antibodies from Abnova produced almost the same results. In addition, χ2 test was done to assess the association between FLJ10540 and osteopontin. As shown in Table 1, FLJ10540 again was significantly associated with osteopontin. Taken together, these results suggest that patients with a high FLJ10540 score had a significantly higher osteopontin score in human NPC.
Immunohistochemical staining for FLJ10540 and osteopontin and overall survival in patients with NPC. (A) The FLJ10540 and osteopontin intensities in various tissues were evaluated by immunohistochemical staining. FLJ10540 and osteopontin immunohistochemical staining intensities are shown in tumor and normal tissues. Normal tissues displayed very weak FLJ10540 and osteopontin staining in the cytoplasm (a, and f). The stainings for FLJ10540 and osteopontin were higher in tumor tissues (b-e and g-j). Significant cytoplasm expressions of FLJ10540 and osteopontin were observed in early (b, and g) and late (c, and h) stage tumors. Original magnification ×100. (B) The survival period of those patients (n=63) with strong expressions of FLJ10540 and osteopontin (dashed lines) were significantly shorter than for those with lower expressions of FLJ10540 and osteopontin (solid line). The difference in survival was statistically significant (p<0.001) according to the log-rank test.
Prognostic value of FLJ10540 and osteopontin expression levels in NPC
As shown in Table 2, patients with stage III-IV tumors, a higher rate of lymph node metastasis, and TNM stages III-IV had significantly higher expressions of FLJ10540 or osteopontin compared with patients with stage I-II tumors (p< 0. 001), with no or a lower rate of lymph node metastasis (p=0. 001 and p= 0. 007), and TNM stages I/II (p< 0. 001). No significant differences between high and low levels of FLJ10540 and osteopontin were observed in terms of the patients’ age, gender, or histological type at the time of diagnosis. These findings suggest that both FLJ10540 and osteopontin expressions correlate with higher tumor progression from an early stage to advanced stage.
A DSS analysis using the Kaplan-Meier methods revealed that the prognosis of patients with high tumor expressions of FLJ10540 and osteopontin were significantly poorer than that of patients with low tumor expressions of FLJ10540 and osteopontin (p< 0. 001) (Figure 2B). Univariate analysis showed that DSS in this series was significantly associated with several clinico-pathological factors, including T (p< 0. 001), N (p= 0. 009), and TNM stage (p= 0. 001) (Table 3). Clinical prognosis, however, was not associated with age, gender or histological type. For multivariate analysis, however, T stage (p< 0. 001; hazard ratio = 5.091) remained significant independent prognostics factor of DSS for NPC patients. These results clearly indicate that the 5-year survival and clinical prognosis of NPC are associated with FLJ10540 and osteopontin expressions in NPC patients.
Osteopontin triggered FLJ10540 expression in NPC cancer cells
As shown in the Figure 3A, the mRNA and protein expression levels of FLJ10540 were increased in an osteopontin dose-dependent manner in TW01 cells by Q-RT-PCR and Western blotting. Similar results were also obtained in the Hone1 cells (Figure 3B). Next, to investigate the role of osteopontin in regulating FLJ10540 transcription, we sought to determine whether FLJ10540 promoter activity could be regulated by osteopontin stimulation. We transfected the FLJ10540 promoter-luciferase constructs into TW01 cells following osteopontin stimulation in a dose-dependent manner. The data showed that a significant activation of the FLJ10540 promoter was detected under osteopontin stimulation (Figure 3C). These results illustrated that FLJ10540 is one of the downstream targets of the osteopontin signaling pathway in human NPC cancer cells.
FLJ10540 was up-regulated by osteopontin stimulation in human cancer cells. (A and B, left panel) The mRNA expression level of FLJ10540 was determined by Q-RT-PCR in TW01, and Hone1 cells with a dose-dependent manner of osteopontin. The results were normalized against the expression level of GAPDH mRNA in each osteopontin-treated cell. (A and B, right panel) The protein expression level of FLJ10540 was determined by Western blotting in TW01, and Hone1 cells. After treatment with various concentrations of osteopontin for 30 min, the total protein was extracted and the cell lysates (50 μg) of each treated cell were subjected to immunoblot analysis with anti-FLJ10540 and β-actin antibodies. β-actin was used as a control. Data are representative of three independent experiments done in triplicate. (C) Luciferase assays were done to detect promoter activity of FLJ10540 in transfected TW01 cells in the presence or absence of osteopontin. The luciferase activity in 1μg of cell lysate was normalized to β-galactosidase activity. Data are representative of three independent experiments done in triplicate.
FLJ10540 transfectants alone or stimulated with osteopontin could promote cell growth in NPC
In Figure 2A, FLJ10540 expression is significantly higher in the NPC patients with a late tumor stage. This raises the possibility that FLJ10540 expression may promote tumor growth in NPC. Two different cell types were employed to address this question. First, TW01 and Hone1 cells stably expressing HA-tagged FLJ10540 were established (Figure 4A and B, left panel). FLJ10540-TW01 stable transfectants or vehicle control cells were seeded in 96-well plates to test whether they could grow in low serum medium. The results showed that FLJ10540-TW01 stable transfectants could promote cell growth in low-serum media compared to the vehicle control (Figure 4A, right panel). Similar results were also observed in FLJ10540-Hone1cells (Figure 4B, right panel). To determine whether osteopontin contributes to FLJ10540-mediated cell growth, FLJ10540-NPC transfectants, and vehicle control in the presence of osteopontin stimulation were seeded in 96-well plates to test cell proliferation. The results showed that FLJ10540-NPC stable transfectants in the presence of osteopontin grew faster than the vehicle control with osteopontin stimulation (Figure 4A and B, right panel). Furthermore, we also found that stimulated FLJ10540 transfectants with osteopontin was also growth faster than FLJ10540 stable cells alone. Taken together, these results suggest that FLJ10540 transfectants with or without osteopontin stimulation may decrease the requirement for serum, a characteristic of transformed cells.
Osteopontin enhanced FLJ10540-NPC transfectants to promote cell proliferation. (A and B, left panel) HA-tagged FLJ10540-stable clones of TW01 and Hone1 cells were established. The cell lysates were subjected to an immunoblot analysis with an anti-HA antibody. (A and B, right panel) Vehicle-TW01/-Hone1 and FLJ10540-TW01/-Hone1 stable transfectants with or without osteopontin (OPN) stimulation were seeded into 96-well plates with 1.0% of FBS. The cells were cultured for 1–3 days followed by MTT assay (OD570) to quantitate cell growth. The data were normalized against the OD570 value on day 1 of each treatment. The growth curve of TW01 and Hone1 cells are shown as the mean ±s.d. of three independent experiments.
Knockdown of endogenous FLJ10540 by siRNA suppressed NPC cell growth
We used a siRNA approach to inhibit endogenous expression of FLJ10540, and assayed for the growth rate of NPC cells. First, we transfected a chemically synthesized FLJ10540 siRNAs (siFLJ10540) and a negative control into TW01 and Hone1 for 24 hours, followed by Western blot analysis using antibodies against FLJ10540. The results showed a dramatic reduction in the FLJ10540 protein level with FLJ10540 siRNA (Figure 5A and B, left panel). Both TW01 and Hone1 siFLJ10540-transfected and negative control were determined the growth rates by MTT assay. The results demonstrated that the proliferation rate of FLJ10540 siRNA transfected cells was much slower than the cells transfected with negative siRNA (Figure 5A and B, right panel). Using the same panel, we also asked whether osteopontin encourages cell growth in depleted-FLJ10540 NPC cells. The result showed that FLJ10540-depleted NPC cells had a slight increased growth curve under osteopontin stimulation, compared to siFLJ10540 alone (Figure 5A and B, right panel). Take together these results strongly supports the idea that FLJ10540 plays a role in the cell proliferation of NPC cells.
Osteopontin reversed the cell growth inhibition of siFLJ10540-NPC. (A and B, left panel) A negative control (NC) siRNA plus one different FLJ10540 siRNA (siFLJ10540) were transfected into TW01 and Hone1 cells for 24 hours. After transfection, Western blotting was performed using anti-FLJ10540 and β-actin antibodies. (A and B, right panels) Using the same panel, the siFLJ10540 transfectants and NC with or without osteopontin (OPN) stimulation were seeded into 96-well plates with 1.0% of FBS. The cells were cultured for 1–3 days followed by MTT assay (OD570) to quantitate cell growth. The data were normalized against the OD570 value on day 1 of each treatment. The growth curve of TW01 and Hone1 cells are shown as the mean ±s.d. of three independent experiments.
FLJ10540 and osteopontin synergistically induced the migration and invasion of NPC cancer cells
FLJ10540 expression was higher in the NPC patients with lymph node metastasis (N+) than those that without lymph node metastasis (N-) (Figure 2A). We hypothesized that FLJ10540 may participate in the progression of metastasis in cells. FLJ10540-TW01 stable cells and vehicle control were seeded on a Transwell chamber to perform a motility assay for migration and invasion. Quantitatively speaking, the migration ability of FLJ10540-TW01 stable transfectants was approximately 4.8-5.5-fold higher than that of the vehicle control (Figure 6A, left panel). We next performed an invasion assay using a Matrigel-coated barrier filter. After 24 hours of incubation in the Matrigel-coated Transwell chamber, the TW01-FLJ10540 transfectants had invaded the basement membrane material and passed through the pores to reach the underside of the filter. Similarly, the TW01-FLJ10540 expressing cells showed a 6.8-7.8-fold higher invasive ability than the vehicle control cells (Figure 6A, right panel). The overexpressed FLJ10540 stable transfectants in the presence of osteopontin stimulation were analyzed the cell motility in a Transwell chamber. Again, FLJ10540 transfectants with osteopontin stimulation had a higher migration and invasion abilities than that the FLJ10540 alone or vehicle control (Figure 6A). Conversely, the motility of the NPC cells was strongly inhibited, while endogenous FLJ10540 was depleted by siRNA (Figure 6B). However, compared with siFLJ10540-NPC cells, the migratory and invasive abilities of siFLJ10540-NPC cells were slightly increased after cell-treated with osteopontin (Figure 6B). Taken together, these results strongly suggest that FLJ10540 is required for proper osteopontin-dependent signaling, and that it contributes to cell migration and invasion in NPC.
FLJ10540 alone or increased FLJ10540, mediated by osteopontin stimulation modulated cell migration and invasion in NPC cells. (A and B) For the migration assays, 5 × 103 cells (Vehicle-TW01, FLJ10540-1-TW01, FLJ10540-2-TW01, negative control, and siFLJ10540-TW01) with or without osteopontin (OPN) stimulation were seeded into the top of a Transwell insert. After 24 hours, the cells on top were scraped, and the cells that had migrated to the bottom were fixed and stained with Giemsa. For the invasion assays, 1 × 104 cells were seeded after the addition of Matrigel. The relative-fold migration/invasion values for the stable clones were normalized against the vehicle/negative control cells and are represented diagrammatically. All of the data represent the mean ± s.d. of three independent experiments.
Osteopontin-mediated FLJ10540 expression promoted cell proliferation and motility via CD44
We next asked whether the involvement of CD44 on the biological activities of osteopontin could be attributed to the modulation of FLJ10540 expression induced by osteopontin in NPC cancer cells. The CD44 receptors were blocked using neutralizing antibodies, and analyzed FLJ10540 protein expression in TW01 cells upon osteopontin stimulation. The results demonstrated that upregulation of FLJ10540 in the presence of osteopontin was inhibited by the simultaneous addition of CD44 antibodies in a dose-dependent manner (Figure 7A). Similar results were also observed in Hone1 cells (data not shown). To confirm the involvement of CD44 in mediating FLJ10540 function upon osteopontin stimulation, we treated FLJ10540-TW01 and vehicle cells with anti-CD44 blocking antibody or isotypes IgG antibody in the presence of osteopontin and monitored cell growth and motility. Anti-CD44 antibodies resulted in a significant inhibition of cell growth and motility by FLJ10540 stable cells in the presence of osteopontin stimulation, compared to FLJ10540 stable cells treated with isotype IgG antibody (Figure 7B, and C). Taken together, these results indicate that FLJ10540 is required for proper osteopontin/CD44-dependent signaling and that it contributes to cell proliferation and metastasis.
CD44 not only participated in the FLJ10540 expression, but also modulated osteopontin/FLJ10540-elicited cell growth and motility in NPC cells. (A) Serum-starved TW01 cells were pre-treated with or without various concentrations of anti-CD44 antibodies for 2 hours; the cells were then stimulated with 30 ng/ml osteopontin for 30 min. The total protein was extracted from TW01 cells and probed with antibodies against FLJ10540. β-actin was used as an internal loading control. (B) For the cell proliferation assay, vehicle-TW01, and FLJ10540-TW01 stable transfectants treated with or without osteopontin stimulation combined with anti-CD44 or IgG antibodies were seeded into 96-well plates with 1.0% FBS. The cells were cultured for 1–3 days followed by MTT assay (OD570) to quantitate cell growth. The data were normalized against the OD570 value on day 1 of each treatment. The growth curve of TW01 cells are shown as the mean ±s.d. of three independent experiments. (C) For the migration and invasion assay, vehicle-TW01 and FLJ10540-TW01 stable clones were pre-treated with or without anti-CD44 or IgG antibodies for 2 hours and seeded into the top of a Transwell insert with or without Matrigel and allowed to adhere for 12 hours. They were then incubated with or without osteopontin (30 ng/ml) for 3 hours. At the end of the assay, the cells on the topside were scraped, and the cells that migrated to the bottom were fixed and stained with Giemsa. All of the data represent the mean ±s.d. of three independent experiments.