Abstract
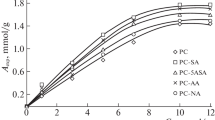
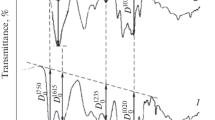
The results of studying the sorption of d-metal cations (Cu2+, Co2+, and Mn2+) from aqueous solutions of their salts by sorbents based on apple pectin modified with organic pharmacophores (salicylic, anthranilic, 5-aminosalicylic, and nicotinic acids) are presented. The influence of the structure of the introduced pharmacophore into the pectin matrix on the kinetics and thermodynamics of the distribution of d‑metal ions in the aqueous-solution–biopolymer-sorbent heterophase system was determined. An increase in the efficiency of extraction of d-metal ions by pharmacophore-containing pectin sorbents was revealed in comparison with the initial polysaccharide. The optimal conditions for the sorption of Cu2+, Co2+, and Mn2+ ions by the studied sorbents (phase contact time, medium pH, temperature) were selected. A mechanism for the interaction of d-metal ions with a modified pectin sorbent has been proposed.




Similar content being viewed by others
REFERENCES
Bush, P.L., Pectin. Chemical Properties. Uses and Health Benefits, New York: Nova Science Publ., 2014.
Sriamornsak, P., Silpakorn Univ. Sci. Int. J., 2003, vol. 3, p. 206.
Thakur, B.R., Singh, R.K., and Handa, A.K., Crit. Rev. Food Sci. Nutr., 1997, vol. 37, p. 47.
Kaisheva, N.Sh. and Kaishev, A.Sh., Pharmakokhimicheskie osnovy primeneniya pektinov i al’ginatov (Pharmacochemical Fundamentals for Application of Pectins and Alginates), Pyatigorsk: RIA-KMV, 2016.
Khotimchenko, Yu.S., Odintsova, M.V., and Kovalev, V.V., Polisorbovit (Polisorbovit), Tomsk: Izd-vo Nauchno-Tekhnicheskoi Literatury, 2001.
Uryash, V.F., Kokurina, N.Yu., Gruzdeva, A.E., et al., Russ. J. Gen. Chem., 2017, vol. 87, no. 13, p. 3212.
Khotimchenko, M.Y., Kolenchenko, E.A., Khotimchenko, Y.S., et al., Colloids Surf., B, 2010, vol. 77, p. 104.
Aleeva, S.V., Chistyakova, G.V., Lepilova, O.V., et al., Russ. J. Phys. Chem. A, 2018, vol. 92, no. 8, p. 1583.
Balaria, A. and Schiewer, S., Sep. Purif. Technol., 2008, vol. 63, no. 3, p. 577.
Kupchik, L.A., Kartel, N.T., Bogdanov, E.S., et al., Russ. J. Appl. Chem., 2006, vol. 79, no. 3, p. 457.
Yamada, M. and Shiiba, S., J. Appl. Polym. Sci., 2015, vol. 132, no. 24, p. 42056.
Gong, J.-L., Wang, X.-Y., Zeng, G.-M., et al., Chem. Eng. J., 2012, vols. 185–186, p. 100.
Zauro, S. and Vashalakshi, B., Sep. Sci. Technol., 2018, vol. 53, no. 14, p. 2170.
Sivagangi, R.N., Rao, K.M., Vani, T.S., et al., Desalin. Water Treat., 2016, vol. 57, no. 14, p. 1.
Li, F.T., Yang, H., Zhao, Y., et al., Chin. Chem. Lett., 2007, vol. 18, p. 325.
Guo, J.J., Zhang, J.Y., Yue, Y., et al., Bulg. Chem. Commun., 2014, vol. 46, p. 801.
Mudarisova, R., Kukovinets, O., Sagitova, A., et al., Biointerface Res. Appl. Chem., 2020, vol. 10, no. 4, p. 5724.
Minzanova, S.T., Chekunkov, E.V., Milyukov, V.A., et al., Dokl. Phys. Chem., 2020, vol. 491, no. 1, p. 24.
Mudarisova, R.Kh., Kukovinets, O.S., Kolesov, S.V., et al., Russ. J. Phys. Chem. A, 2021, vol. 95, no. 9, p. 1835.
Aqdas, N., Zill-i-Huma, N., Javeria, A., et al., Int. J. Biol. Macromol., 2017, vol. 101, p. 254.
Wang, R., Liang, R., and Dai, T., Trends Food Sci. Technol., 2019, vol. 91, p. 319.
Bhuyan, M., Okabe, H., Hidaka, Y., et al., J. Appl. Polym. Sci., 2018, vol. 135, p. 45906.
Sharma, P., Sen, K., Thakur, P., Chauhan, M., and Chauhan, K., Int. J. Biol. Macromol., 2019, vol. 140, p. 78.
Sagitova, A.F., Mudarisova, R.Kh., Kukovinets, O.S., and Akhmetshina, L.I., Bull. Bashk. Univ., 2018, vol. 23, no. 2, p. 323.
Liang, R., Li, Y., Huang, Li, et al., Carbohydr. Polym., 2020, vol. 234, p. 115911.
Hang, P.T. and Brindley, G.W., Clays Clay Miner., 1970, vol. 18, p. 203.
Rafikov, S.R., Budtov, V.T., and Monakov, Yu.B., Vvedenie v fiziko-khimiyu rastvorov polimerov (Introduction to the Physical Chemistry of Polymer Solutions), Moscow: Nauka, 1978.
Donchenko, L.V., Tekhnologiya pektinov i pektinoproduktov (Technology of Pectins and Pectin Products), Moscow: DeLi, 2000.
Albert, A. and Serjeant, E.P., Ionization Constants of Acids and Bases, A Laboratory Manual, London, Methuen, New York: Wiley, 1962.
Zolotov, Yu.A., Osnovy analiticheskoi khimii. Prakticheskoe rukovodstvo (Fundamentals of Analytical Chemistry. Practical Guide), Moscow: Vysshaya Shkola, 2001.
Korenman, I.M., Novye titrimetricheskie metody (New Titrimetric Methods), Moscow: Khimiya, 1983.
Hawari, A., Rawajfih, Z., and Nsour, N., J. Hazard. Mater., 2009, vol. 168, p. 1284.
Koksharov, S.A., Aleeva, S.V., and Lepilova, O.V., J. Mol. Liq., 2019, vol. 283, p. 606.
Nikiforova, T.E. and Kozlov, V.A., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, no. 3, p. 399.
Khotimchenko, M.Y., Kolenchenko, E.A., and Khotimchenko, Yu.S., J. Colloid Interface Sci., 2008, vol. 323, p. 216.
Nikiforova, T.E., Kozlov, V.A., and Sionikhina, A.N., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 5, p. 849.
Lia, J., Yang, Z., Ding, T., et al., Carbohydr. Polym., 2022, vol. 276, p. 118789.
Farooq, U., Kozinski, J., Mishabul, A., et al., Bioresour. Technol., 2010, vol. 101, p. 5043.
Wang, R., Liang, R., and Dai, T., Trends Food Sci. Technol., 2019, vol. 91, p. 319.
Mudarisova, R.Kh., Sagitova, A.F., Kukovinets, O.S., et al., Russ. J. Gen. Chem., 2020, vol. 90, no. 4, p. 660.
ACKNOWLEDGMENTS
Analyses (measurements and calculations) were performed using the equipment of the “Chemistry” Core Facilities Center of the Ufa Institute of Chemistry of the Russian Academy of Sciences and the “Agidel” Regional Core Facilities Center of the Ural Federal Research Center of the Russian Academy of Sciences.
Funding
The article was prepared as part of the implementation of the program of fundamental scientific research of state academies for 2013–2020, state order no. 1021062311391-0-1.4.4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Mudarisova, R.K., Sagitova, A.F. & Kukovinets, O.S. Analysis of Sorption Activity of Apple Pectin Modified with Organic Pharmacophores in Relation to d-Metal Cations (Cu2+, Co2+, and Mn2+). Prot Met Phys Chem Surf 58, 927–934 (2022). https://doi.org/10.1134/S2070205122050173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205122050173