Abstract
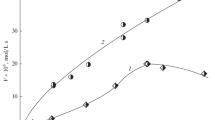
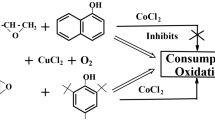
The oxidation of the epoxide–chloride Cu (II)–an aromatic alcohol ternary system containing an alicyclic series epoxide derivative is studied for the first time. The concentration dependence of the rate of oxygen uptake by the CE–CuCl2–IOL ternary system, where CE is cyclohexene epoxide and IOL is ionol, is expressed as V = k [CE]0 [CuCl2]0.6[IOL]0.7 for [CE] \( \gg \) [IOL] > [CuCl2] in a methanol solution. The Arrhenius dependence of the effective oxidation rate constant in the temperature range 313–328 K has the form k = 8.0 × 106 exp (–54.0/RT). A reaction mechanism based on the copper chloride-catalized electron transfer from the OH-bond of the substituted phenol to the C–O bond of the epoxide with the formation of an oxyalkyl radical is proposed. The free radical yield, estimated from the ratio of the rate of oxygen uptake to the rate of epoxide consumption, is ~50%.





Similar content being viewed by others
REFERENCES
R. E. Parker and N. S. Isaacs, Chem. Rev. 59, 737 (1959).
L. V. Petrov and V. M. Solyanikov, Pet. Chem. 39, 89 (1999).
M. G. Spirin, S. B. Brichkin, and L. V. Petrov, Russ. Chem. Bull. 65, 2452 (2016).
L. V. Petrov and V. M. Solyanikov, Pet. Chem. 45, 202 (2005).
L. V. Petrov and V. M. Solyanikov, Russ. J. Phys. Chem. B 14, 29 (2020).
L. V. Petrov and V. M. Solyanikov, Russ. Chem. Bull. 69, 1869 (2020).
L. V. Petrov and V. M. Solyanikov, Russ. J. Phys. Chem. B 15, 599 (2021).
V. D. Pokhodenko, L. S. Degtyarev, V. G. Koshechko, and V. S. Kuts, Free Radical Chemistry Problems (Nauk. Dumka, Kiev, 1984), p. 110 [in Russian].
E. T. Denisov, The Rate Constants of Homolytic Liquid-Phase Reactions (Nauka, Moscow, 1971), p. 328 [in Russian].
C. L. Jenkins and J. K. Kochi, J. Org. Chem. 36, 3103 (1971).
N. K. Naumenko, Extended Abstract of Cand. Sci. (Chem.) Dissertation (Lensovet Leningr. Tech. Inst., Leningrad, 1970).
Funding
This study was carried out on the topic of a state assignment (registration number AAAA-A19-119071890015-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrov, L.V., Solyanikov, V.M. Oxidation with Molecular Oxygen of the Cyclohexene Epoxide–Copper(II) Chloride–Ionol Ternary System. Russ. J. Phys. Chem. B 15, 960–964 (2021). https://doi.org/10.1134/S1990793121060075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121060075