Abstract
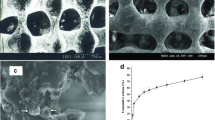
Mechanical loading influences on the chondrocyte and may promote articular cartilage repair in vivo. That is thought to play a key role in the differentiation of MSCs and in tissue healing and repair. We aimed to evaluate the effect of mechanical Loading of walking on articular cartilage repair. So the stem cells were isolated from the infrapatellar adipose tissue and were seeded on PCL. Then cell-scaffold constructs were sputter-coated with gold and observed by scanning electron microscopy to determine the adhesion of ASCs on the scaffold. ASCs-PCL constructs were transplanted into defects in sheep knees then ASCs on the scaffold was induced by the mechanical loading in vivo. Repaired tissue was evaluated with Q(RT-PCR), immunofluorescence staining, toluidine blue staining and Masson’s trichrome staining, the results revealed the largest areas of type II collagen staining, Sox9 expression, aggrecan and presence of chondrocytes in repaired cartilage in experimental groups. Also, the results of this study suggest that mechanical loading of walking could be used to induce ASCs to repair articular cartilage in vivo.







Similar content being viewed by others
REFERENCES
Aisenbrey, E.A. and Bryant, S.J., Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel, J. Mater. Chem. B., 2016, vol. 4, pp. 3562–3574.
Anthony, R. M., Jay, M.P., Hannah, M. Z., James, L. C., and Robert, L. M., Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations, NPJ Regener. Med., 2019, vol. 4, pp.12–18. https://doi.org/10.1038/s41536-019-0074-7
Cheng, C., Dhananjay, T. T., Linhong, and D., Liu, Y., Biomechanical properties and mechanobiology of the articular chondrocyte, Am. J. Physiol. Cell Physiol., 2013, vol. 305, pp. 1202C–C1208.
Carroll, S.F., Buckley, C.T., Kelly, and D.J., Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad, J. Biomech., 2014, vol. 47, pp. 2115–2121.
Chung, J.Y., Song, M., Ha, C., Kim, J., Lee, C., and Park, Y., Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model, Stem Cell Res Ther., 2014, vol. 5, pp. 1–13.
Cao, W., Lin, W., Cai, H., Chen, Y., Man, Y., Liang, J., Wang, Q., Sun, Y., Fan, Y., and Zhang, X., Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds, Regener. Biomat., 2019, vol. 22, pp. 99–106.
Elamparithi, A., Punnoose, A.M., Kuruvilla, S., Ravi, M., Rao, S., and Paul, S., Electrospun polycaprolactone matrices with tensile properties suitable for soft tissue engineering, Artif. Cells Nanomed. Biotechnol., 2016, vol. 44, pp. 878–884.
Fitzgerald, J., Endicott, J., Hansen, U., and Janowitz, C., Articular cartilage and sternal fibrocartilage respond differently to extended microgravity, NPJ Microgravity, 2019, vol. 5, pp. 143–52.
Gaut, C. and Sugaya, K., Critical review on the physical and mechanical factors involved in tissue engineering of cartilage, Regener. Med., 2015, vol.10, pp. 665–679.
Guo,T., Yu, L., Lim, C.G., Goodley, S.A., **ao, X., Placone, K.J., Ferlin, M.K., Nguyen, B.B., Hsieh, H.A and Fisher, P.J., Effect of dynamic culture and periodic compression on human mesenchymal stem cell proliferation and chondrogenesis, Ann. Biomed. Eng., 2016, vol. 44, pp. 2103–2113.
Gahunia, H.K. and Pritzker, K., Effect of Exercise on articular cartilage, Orthop. Clin. North Am.,2012, Vol.43, pp. 187–199.
Hannes, Z., Eugenia, N., Florian, H., Christoph, B., and Vivek, J., Correlation analysis of SOX9, -5, and -6 as well as COL2A1 and aggrecan gene expression of collagen I implant-derived and osteoarthritic chondrocytes, Cartilage, 2016, vol. 7, pp. 185–192.
Iseki,T., Rothrauff, B., Kihara, S., Sasaki, H., Yoshiya, H., FU, F., Tuan, S., and Gottardi, R., Dynamic compressive loading improves cartilage repair in an in vitro model of microfracture, Am. J. Sports Med., 2019, vol. 47, pp. 2188–2199.
Jiang, X., Liu, J., and Liu, Q., Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes, Biomater. Sci., 2018, vol. 6, pp.1616–1626.
Kalarestaghy, H., Shafaei, H., Farahzadi, R., Vahedi, P., Dolathkhah, M.A., Del Azar, A., and Nahid Karimian, N., Collagen I gel increases the osteogenic potential of platelet-rich plasma in adipose-derived stem cells, Crescent J. Med. Biol. Sci., 2020, vol. 7, pp. 373–381.
Li, L., Newton, P.T., Bouderlique, T., Sejnohova, M., Zikmund, T., Kozhemyakina, E., **e, M., Krivanek, J., Kaiser, J., Qian, H., Dyachuk, V., Lassar, A., Warman M., Barenius, B., Adameyko, I., and Chagin, A., Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice, Faseb. J., 2017, vol. 31, pp. 1067–1084.
Liu, C.F. and Lefebvre, V., The transcription factors Sox9 and Sox5/Sox6 cooperate genome-wide through super-enhancers to drive chondrogenesis, Nucleic Acids Res., 2015, vol. 43, pp.8183–8203.
Madeira, C., Santhagunam, A., Salgueiro, J.B., and Cabral, J.M., Advanced cell therapies for articular cartilage regeneration, Trends Biotechnol., 2015, vol. 33, pp. 35–42.
Montaseri, A., Roshangar, L., Rad, JS., Shafaei, H., and Shakibaei, M., Formation of repaired hyaline cartilage using PDGF-treated chondrocyte PCL construct in rabbit knee articular cartilage defect, Ann. Biol. Res., 2019, vol. 3, pp. 32–40.
Niamh, F., Mauro, A., and Martin, J., Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering, J. Orthopedic Res., 2017, vol. 5, pp. 1–34.
Popryadukhin, P.V., Popov, G. I., Yukina, G. Yu., Dobrovolskaya, I.P., Ivan’kova, E.M., Vavilov, V.N., and Yudin, V.E., Tissue-engineered vascular graft of small diameter based on electrospun polylactide microfibers. Int. J. Biomaterials., 2017, article ID 9034186.
Pot, M.W., Kuppevelt, T., Gonzales, V., Buma, P., IntHout, J., Vries, R., and Daamen, W., Augmented cartilage regeneration by implantation of cellular versus acellular implants after bone marrow stimulation: A systematic review and meta-analysis of animal studies, Peer J., 2017, vol. 5, p. e3927.
Shim, J.H., Jang, K.M., Hahn, S.K., Park, J.Y., Jung, H., Oh, K., Park, K., Yeom, J., Park, S., Kim, S., Wang, J., Kim, K., and Cho, D., Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint, Biofabrication, 2016, vol. 8, p. 014102.
Tan, A.R. and Hung, C.T., Concise review: Mesenchymal stem cells for functional cartilage tissue engineering: Taking cues from chondrocyte-based constructs, Stem Cells Trans. Med., 2017, vol. 4, pp. 321–329.
Vega, S., Kwon, M., and Burdick, J., Recent advances in hydrogels for cartilage tissue engineering, Eur. Cells Mater., 2017, vol. 2, pp. 33–59.
Xue, R., Qian, Y., Li, L., Yao, G., Yang, L and Sun, Y., Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells, Stem Cell Res. Ther., 2017, vol. 8, pp. 148s–156s.
Zhang, Z.,WU, S., Naccarato, T., Manan Prakash-Damani, M., Chou,Y ., Chu, C., and Zhu,Y ., Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells, PLoS One, 2017, vol. 12, p. e0180138. https://doi.org/10.1371/journal
Zhang, S., Chen, X., Hu, Y., Wu, J.,Cao, Q., Chen, S., and Gao, Y., All-trans retinoic acid modulates Wnt3A-induced osteogenic differentiation of mesenchymal stem cells via activating the PI3K/AKT/GSK3beta signalling pathway, Mol. Cell Endocrinol., 2016, vol. 422, pp. 243–253.
Funding
This work was carried out with financial support of Maragheh University of Medical Sciences, project no. IR.MARAGHEH.REC.1397.010. and the authors would like to thank Maragheh University of Medical Sciences, Maragheh, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.Statement on the welfare of animals. Animal experiments were carried out in accordance with generally accepted ethical international standards in compliance with the principles of humaneness set out in the European Community Directive (2010/63/EC) and the ethical committee (IR.MARAGHEHPHC.REC.1397.5) of Maragheh University of Medical Sciences approved all housing conditions and experiments.
Additional information
Abbreviations: Q(RT-PCR)—quantitative real-time polymerase chain reaction; ASCs—adipose tissue derived stem cell; PCL—poly (ε-caprolactone); COLII—collagen type 2; COMP—cartilage oligomeric matrix protein; SOX9—SRY-box transcription factor; GAGs—glycosaminoglycans; TGF-b—transforming growth factor beta; iPSCs—pluripotent stem cells.
Rights and permissions
About this article
Cite this article
Vahedi, P., Jarolmasjed, S.H. & Soleimani, A. Transplantation of ASCs-Poly (ε-Caprolactone) Nanofiber Scaffold and Evaluate the Effect of Mechanical Loading of Walking on Articular Cartilage Repair in Sheep Model. Cell Tiss. Biol. 15, 199–207 (2021). https://doi.org/10.1134/S1990519X21020115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X21020115