Abstract
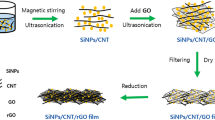
Two types of treatment of the initial mechanical mixture [silicon nanopowder and graphene oxide (GO)] for obtaining Si/RGO nanocomposites were used: reduction in hydrazine vapor and heat treatment at 550°C in an argon atmosphere. It was shown that the type of reduction has an influence on the morphological and electrochemical characteristics of the composites due to the formation of defects and the presence of nitrogen in the graphene network. Less defective and nitrogen doped Si/RGO composites have a better electrochemical behavior as an active material of negative electrode for lithium-ion batteries. The discharge capacity of electrodes based on Si/RGO nanocomposites amounted to 437 mA h g–1 without polymer binder and 1192 mA h g–1 with CMC as a binder.






Similar content being viewed by others
REFERENCES
Lu, J., Chen, Z., Pan, F., et al., Electrochem. Energy Rev., 2018, vol. 1, pp. 35–53. https://doi.org/10.1007/s41918-018-0001-4
Obrovac, M.N. and Christensen, L. Electrochem. Solid State Lett., 2004, vol. 7, no. 5, pp. A93–A96.
Luo, F., Liu, B., Zheng, J., et al., J. Electrochem. Soc., 2015, vol. 162, no. 14, pp. A2509–A2528. https://doi.org/10.1149/2.0131514jes
Wu, H., Cui, Y. Nano Today, 2012, vol. 7, no. 5, pp. 414–429.
Evshchik, E.Yu., Korchun, A.V., Levchenko, A.V., et al., Int. J. Electrochem. Sci., 2021, vol. 16, no. 1, p. 151035
Choi, J.W. and Aurbach, D., Nat. Rev. Mater., 2016, vol. 1, no. 4, pp. 1–16.
Dobrovolsky, Yu.A., Bushkova, O.V., Astafiev, E.A., et al., Lithium-Ion Batteries: Textbook. Allowance, Moscow: RCTU named after D.I. Mendeleev, 2020.
Zhou, X., Yin, Y.-X., Wan, L.-J., et al., Adv. Energy. Mater., 2012, vol. 2, no. 9, pp. 1086–1090.
Ding, N., Chen, Y., Li, R., et al., Electrochim. Acta, 2020, vol. 367, no. 20, pp. ID 137491. https://doi.org/10.1016/j.electacta.2020.137491
Agyeman, D.A., Song, K., Lee, G.-H., et al., Adv. Energy Mater., 2016, vol. 6, no. 20, pp. 1600904.
Botas, C., Carriazo, D., Zhang, W., et al., ACS Appl Mater Interfaces, 2016, vol. 8, no. 42, pp. 28800–28808.
Korchun, A.V., Evshchik, E.Yu., Baskakov, S.A., Bushkova, O.V., Dobrovolsky, Y.A. Chimica Techno Acta, Forthcoming 2020. https://doi.org/10.15826/chimtech.2020.7.4.21
Manzhos, R., Baskakov, S., Kabachkov, E., et al., Materials, 2021, vol. 14, p. 322. https://doi.org/10.3390/ma14020322
Novikov, D.V., Evschik, E.Yu., Berestenko, V.I., et al., Electrochimica Acta, 2016, vol. 208, pp. 109–119.
Shulga, Yu.M., Kabachkov E.N., Baskakov S.A., et al., Russ. J. Phys. Chem. A, 2019, vol. 93, no. 2, pp. 296–300. https://doi.org/10.1134/S0036024419010278
Lin, Q., Zhang, J., Kong, D., et al., Adv. Energy Mater., 2018, vol. 9, p. 1803078. https://doi.org/10.1002/aenm.201803078
Wu, Z.-S., Ren, W., Xu, L., et al., ACS Nano, 2011, vol. 5, no. 7, pp. 5463-5471.
Li, X., Geng, D., Zhang, Y., et al., Electrochem Communications, 2011, vol. 13, pp. 822–825.
Wang, H., Zhang, C., Liu, Z., et al., J. Mater. Chem., 2011, no. 21, pp. 5430–5434.
Reddy, A.L.M., Srivastava, A., Gowda, S.R., et al., ACS Nano, 2010, vol. 4, no. 11, pp. 6337–6342.
Wei, D., Liu, Y., Wang, Y., et al., Nano Lett., 2009, vol. 9, no. 5, pp. 1752–1758.
ACKNOWLEDGMENTS
This work was performed with financial support from the Ministry of Science and Higher Education of Russian Federation, project ID RFMEFI60419X0235.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest requiring disclosure in this paper.
Rights and permissions
About this article
Cite this article
Korchun, A.V., Evshchik, E.Y., Baskakov, S.A. et al. Silicon-Reduced Graphene Oxide Composite as Negative Electrode of Li-Ion Batteries. Russ J Appl Chem 93, 1940–1946 (2020). https://doi.org/10.1134/S1070427220120174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220120174