Abstract
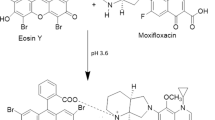
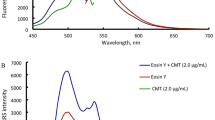
A simple, fast, sensitive, cost-effective, and well-proven spectrofluorimetric method has been developed for the quantification of cetirizine dihydrochloride (CTZ). The quenching effect of CTZ on the fluorescence intensity of Eosin Y was utilized to generate an ion-pair complex that can be detected at 549 nm using a 301 nm excitation wavelength in the presence of glycine buffer (pH 4.0) for the method development. The factors that influence reactions were thoroughly examined and optimized. With a determination coefficient of 0.9996, the fluorescence quenching value was linear to CTZ concentration in the range of 1–40 µg/mL. The estimated detection and quantification limits were determined to be 0.02 and 0.08 µg/mL, respectively. The method selectivity was validated by analyzing the effects of excipients, and no interference was found. The established approach was applied to determine the presence of CTZ in products available on the market as well as in biological samples. The methodology was validated using guidelines from the International Conference on Harmonization, and percent recoveries for pharmaceutical items varied from 90.6 to 103.5% and for biological fluids from 97.9 to 102.5%. The method has been successfully used for the content uniformity test with high percent recovery and low relative standard deviation.










Similar content being viewed by others
REFERENCES
Corsico, A.G., Leonardi, S., Licari, A., Marseglia, G., Miraglia del Giudice, M., Peroni, D.G., and Ciprandi, G., Multidiscip. Respir. Med., 2019, vol. 14, p. 40.
Bajerski, L., Sangoi, M.D.S., Barth, T., Diefenbach, I.F., Dalmora, S.L., and Cardoso, S.G., Quim. Nova, 2010, vol. 33, no. 1, p. 114.
Leistner, A., Haerling, S., Kreher, J.D., Becker, I., Jung, D., and Holzgrabe, U., J. Pharm. Biomed. Anal., 2020, vol. 189, p. 113425.
Ibrahim, F., Sharaf El-Din, M.K., Eid, M., and Wahba, M.E.K., Int. J. ChemTech Res., 2011, vol. 2, no. 8, p. 2056.
Jaber, A.M.Y., Al Sherife, H.A., Al Omari, M.M., and Badwan, A.A., J. Pharm. Biomed. Anal., 2004, vol. 36, no. 2, p. 341.
El-Kommos, M.E., El-Gizawy, S.M., Atia, N.N., and Hosny, N.M., Anal. Chem. Res., 2015, vol. 3, p. 1.
Patil, R.H., Hegde, R.N., and Nandibewoor, S.T., Colloids Surf., B., 2011, vol. 83, no. 1, p. 133.
Aly, F.A., Nahed, E.E., Elmansi, H., and Nabil, A., Chem. Cent. J., 2017, vol. 11, p. 99.
Karakuş, S., Küçükgüzel, İ., and Küçükgüzel, Ş.G., J. Pharm. Biomed. Anal., 2008, vol. 46, no. 2, p. 295.
Ulu, S.T., J. Food Drug Anal., 2010, vol. 18, no. 6, p. 440.
Bhatia, N.M., Ganbavale, S.K., and More, H.N., Asian J. Pharm., 2008, vol. 2, no. 3.
Rahman, H., Int. J. Pharm. Pharm. Sci., 2017, vol. 9, p. 1.
Khan, M.N., Irum, and Mursaleen, M., Luminescence, 2021, vol. 36, no. 2, p. 515.
Derayea, S.M., Gahlan, A.A., Omar, M.A., Saleh, G.A., and Haredy, A.M., Luminescence, 2020, vol. 35, no. 7, p. 1028.
Kabra, P., Nargund, L.V.G., and Murthy, M.S., Trop. J. Pharm. Res., 2014, vol. 13, no. 7, p. 1141.
Dhongle, P.S., Sahare, S.J., Dhongle, S.S., Mundhey, A.S., and Wate, S.P., Res. J. Pharm. Technol., 2011, vol. 4, no. 9, p. 1471.
El-Didamony, A.M. and Ramadan, G.M., SN Appl. Sci., 2020, vol. 2, no. 4, p. 723.
Williams, R.L., Adams, W.P., Poochikian, G., and Hauck, W.W., Pharm. Res., 2002, vol. 19, no. 4, p. 359.
The United States Pharmacopeia 30, the National Formulary 25, Rockville: US Pharmacopeial Convention, 2007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shah Mansoor, Jan, F.A. & Ullah, N. Determination and Quantification of Cetirizine Dihydrochloride in Pharmaceutical Formulations and Biological Fluids Through Fluorescence Quenching of Eosin Y: Application to Content Uniformity Test. J Anal Chem 78, 965–974 (2023). https://doi.org/10.1134/S1061934823080129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934823080129