Abstract
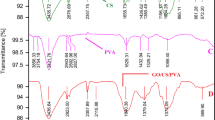
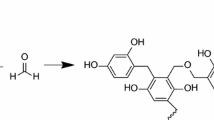
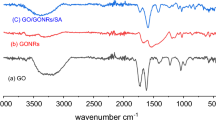
The article proposes a method for producing polyaniline-modified nanocomposite cryogel based on oxidized carbon nanotubes and reduced graphene oxide. Phenol–formaldehyde resin has been used as a crosslinking agent. Cryogel has been obtained by freeze drying in vacuum. Then, the material has been subjected to a post-processing, i.e., the carbonization in a tubular furnace. The obtained nanocomposite has been subjected to the comprehensive diagnostics by the methods of scanning and transmission electron microscopy, IR spectroscopy, X-ray diffraction analysis, and Raman spectroscopy. The parameters of the pore space have been estimated by nitrogen adsorption. It has been found that the carbonized nanocomposite cryogel is a mesoporous material with a specific surface area of 299 m2/g. IR and Raman spectra and X-ray diffraction patterns of the starting materials have been compared with the spectra of the carbonized cryogel. According to the results obtained, the nanocomposite exhibits peaks of all starting materials. The sorption capacity of the material has been evaluated by the example of the sorption of ions of a heavy metal, lead, from model aqueous solutions. Kinetic studies of adsorption in a limited volume have been carried out to determine the mechanism and time of the adsorption. It has been revealed that 99% of the contaminant is sorbed during the first 15 min, while an adsorption capacity of 295 mg/g is reached. The Elovich model, pseudo-first- and pseudo-second-order models, and an intradiffusion model have been employed to confirm the proposed adsorption mechanism.









Similar content being viewed by others
REFERENCES
Burakov, A.E., Galunin, E.V., Burakova, I.V., et al., Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review, Ecotoxicol. Environ. Saf., 2018, vol. 148, pp. 702–712. https://doi.org/10.1016/j.ecoenv.2017.11.034
Kadum, A.H.K., Burakova, I.V., Mkrtchyan, E.S., et al., Sorption kinetics of organic dyes methylene blue and malachite green on highly porous carbon material, J. Adv. Mater. Technol., 2023, vol. 8, no. 2, pp. 130–140. https://doi.org/10.17277/jamt.2023.02.pp.130-140
Gupta, K., Joshi, P., Gusain, R., and Khatri, O.P., Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials, Coord. Chem. Rev., 2021, vol. 445, p. 214100. https://doi.org/10.1016/j.ccr.2021.214100
Jan, A., Azam, M., Siddiqui, K., et al., Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants, Int. J. Mol. Sci., 2015, vol. 16, no. 12, pp. 29592–29630. https://doi.org/10.3390/ijms161226183
Zwolak, A., Sarzyńska, M., Szpyrka, E., and Stawarczyk, K., Sources of soil pollution by heavy metals and their accumulation in vegetables: A review, Water, Air, Soil Pollut., 2019, vol. 230, no. 164, pp. 1–9. https://doi.org/10.1007/s11270-019-4221-y
SanPiN 2.1.4.1074-01, Drinking water, hygienic requirements for water quality in centralized drinking water supply systems, quality control, hygienic requirements for ensuring the safety of hot water supply systems. https://files.stroyinf.ru/Data1/9/9742/index.htm. Accessed on November 30, 2023.
Heavy metals in water: Content, identification and analysis. https://ion-lab.ru/tyazhelyie-metallyi-v-vode/. Accessed on November 30, 2023.
Chen, Y., Xu, F., Li, H., et al., Simple hydrothermal synthesis of magnetic MnFe2O4-sludge biochar composites for removal of aqueous Pb2+, J. Anal. Appl. Pyrolysis, 2021, vol. 156, p. 105173. https://doi.org/10.1016/j.jaap.2021.105173
Ding, X., Yang, S., Zhou, S., et al., Biomimetic molecule catalysts to promote the conversion of polysulfides for advanced lithium–sulfur batteries, Adv. Funct. Mater., 2020, vol. 30, no. 38, p. 2003354. https://doi.org/10.1002/adfm.202003354
Li, Y., Dong, X., and Zhao, L., Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water, Int. J. Biol. Macromol., 2021, vol. 192, no. 1, pp. 118–125. https://doi.org/10.1016/j.ijbiomac.2021.09.202
Barus, D.A., Humaidi, S., Ginting, R.T., and Sitepu, J., Enhanced adsorption performance of chitosan/cellulose nanofiber isolated from durian peel waste/graphene oxide nanocomposite hydrogels, Environ. Nanotechnol., Monit. Manage., 2022, vol. 17, p. 100650. https://doi.org/10.1016/j.enmm.2022.100650
Ali, I., Galunin, E.V., Burakov, A.E., and Mkrtchyan, E., High-speed and high-capacity removal of methyl orange and malachite green in water using newly developed mesoporous carbon: Kinetic and isotherm studies, ACS Omega, 2019, vol. 4, pp. 19293–19306. https://doi.org/10.1021/acsomega.9b02669
Birniwa, A.H., Ali, U., Jahun, B.M., and Hayatu Mustapha, M., Cobalt oxide doped polyaniline composites for methyl orange adsorption: Optimization through response surface methodology, Case Stud. Chem. Environ. Eng., 2024, vol. 9, p. 100553. https://doi.org/10.1016/j.cscee.2023.100553
Ben, S.K., Gupta, S., Raj, K.K., and Chandra, V., Adsorption of malachite green from polyaniline facilitated cobalt phosphate nanocomposite from aqueous solution, Chem. Phys. Lett., 2023, vol. 820, p. 140469.
Liu, W., Lou, T., and Wang, X., Enhanced dye adsorption with conductive polyaniline doped chitosan nanofibrous membranes, Int. J. Biol. Macromol., 2023, vol. 242, no. 1, p. 124711. https://doi.org/10.1016/j.ijbiomac.2023.124711
Khan, N.A., Hassan, M., Lee, H.J., and Jhung, S.H., Highly porous polyaniline- or polypyrrole-derived carbons: Preparation, characterization, and applications in adsorption, Chem. Eng. J., 2023, vol. 474, p. 145472. https://doi.org/10.1016/j.cej.2023.145472
Ali, I., Kuznetsova, T.S., Burakov, A.E., Burako-va, I.V., Pasko, T.V., Dyachkova, T.P., Mkrt-chyan, E.S., Babkin, A.V., Tkachev, A.G., Albishri, H.M., Alshitari, W.H., Hameed, A.M., and Alharbi, A., Polyaniline modified CNTs and graphene nanocomposite for removal of lead and zinc metal ions: Kinetics, thermodynamics and desorption studies, Molecules, 2022, vol. 27, no. 17, p. 5623. https://doi.org/10.3390/molecules27175623
Lagergren, S.K., About the theory of so-called adsorption of soluble substances, Sven. Vetenskapsakad. Handingarl, 1898, vol. 24, pp. 1–39.
Ho, Y.S. and McKay, G., Sorption of dye from aqueous solution by peat, Chem. Eng. J., 1998, vol. 70, no. 2, pp. 115–124. https://doi.org/10.1016/S1385-8947(98)00076-X
Elovich, S.Y. and Larinov, O.G., Theory of adsorption from solutions of non-electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions, Izvestiya Akademii Nauk. SSSR, 1962, vol. 2, pp. 209–216.
Weber, W. and Morris, J., Intraparticle diffusion during the sorption of surfactants onto activated carbon, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, vol. 89, no. 1, pp. 53–61.
Boyd, G.E., Schubert, J., and Adamson, A.W., The exchange adsorption of ions from aqueous solutions by organic zeolites. Ion-exchange equilibria, J. Am. Chem. Soc., 1947, vol. 69, no. 11, pp. 2818–2829. https://doi.org/10.1021/ja01203a064
Funding
This work was supported by the Russian Science Foundation (project no. 22-13-20074), https://rscf.ru/project/22-13-20074.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by A. Kirilin
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuznetsova, T.S., Burakov, A.E., Ananyeva, O.A. et al. Kinetics of Lead Sorption from Aqueous Solutions on Nanostructured Cryogel Modified with Organic Polymers. Colloid J (2024). https://doi.org/10.1134/S1061933X24600131
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1061933X24600131