Abstract
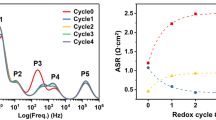
The kinetics of nickel reduction and morphological changes in Ni–10Sc1CeSZ composite anodes in intermediate-temperature solid oxide fuel cells (SOFC) are studied using the Raman spectroscopy technique with the help of application of optically transparent single crystal solid electrolyte membranes and also the thermogravimetric analysis technique. It is shown that the first reduction cycle differs considerably from all the further ones, which is related to morphological changes of nickel grains occurring during the first reduction cycle. A general scheme of occurrence of the process is suggested in studies of model cells using the Raman spectroscopy technique and also in the case of thermogravimetric analysis of powders; it explains the causes for significant differences between the total duration of the process as measured using different techniques. The results of the work can be used for optimization of the mode of initial reduction of the anodic SOFC electrode.
Similar content being viewed by others
References
Wachsman, E.D. and Lee, K.T., Science, 2011, vol. 334, p. 935.
Huijsman, J.P.P., van Berkel, F.P.F., and Christie, G.M., J.Power Sources, 1998, vol. 71, p. 107.
Dusastre, V. and Kilner, J.A., Solid State Ionics, 1999, vol. 126, p. 163.
Fu, C., Sun, K., Zhang, N., Chen, X., and Zhou, D., Electrochim. Acta, 2007, vol. 52, p. 4589.
Nielsen, J., Jacobsen, T., and Wandel, M., Electrochim. Acta, 2011, vol. 56, p. 7963.
Rembelski, D., Viricelle, J.P., Combemale, L., and Rieu, M., Fuel Cells, 2012, vol. 12, p. 256.
Hecht, E.S., Gupta, G.K., Zhu, H., Dean, A.M., Kee, R.J., Maier, L., and Deutschmann, O., Appl. Catal., A, 2005, vol. 295, p. 40.
Lee, J.-H., Moon, H., Lee, H.-W., Kim, J., Kim, J.-D., and Yoon, K.-H., Solid State Ionics, 2002, vol. 148, p. 15.
Koide, H., Someya, Y., Yoshida, T., and Maruyama, T., Solid State Ionics, 2000, vol. 132, p. 253.
Ishihara, T., Yan, J., Shinagawa, M., and Matsumoto, H., Electrochim. Acta, 2006, vol. 52, p. 1645.
Ju, Y.-W., Eto, H., Inagaki, T., Ida, S., and Ishihara, T., J.Power Sources, 2010, vol. 195, p. 6294.
Ju, Y.-W., Ida, S., Inagaki, T., and Ishihara, T., J.Power Sources, 2011, vol. 196, p. 6062.
Agarkov, D.A., Burmistrov, I.N., Tsybrov, F.M., Tartakovskii, I.I., Kharton, V.V., Bredikhin, S.I., and Kveder, V.V., ECS Trans., 2015, vol. 68, p. 2093.
Borik, M.A., Lomonova, E.E., Osiko, V.V., Panov, V.A., Porodnikov, O.E., Vishnyakova, M.A., and Koron’ko, Yu.K., J.Cryst. Growth, 2005. vol. 275, p. e2173.
Aleksandrov, V.I., Osiko, V.V., Prokhorov, A.M., and Tatarintsev, V.M., Russ. Chem. Rev., 1978, vol. 47, p. 213.
Artemov, V.G., Kuritsyna, I.E., Lebedev, S.P., Komandin, G.A., Karpalov, P.O., Spektor, I.E., Kharton, V.V., Bredikhin, S.I., and Volkov, A.A., Russ. J. Electrochem., 2014, vol. 50, p. 690.
Burmistrov, I., Agarkov, D., Bredikhin, S., Nepochatov, Yu., Tiunova, O., and Zadorozhnaya, O., ECS Trans., 2013, vol. 57, p. 917.
Burmistrov, I.N., Agarkov, D.A., Tartakovskii, I.I., Kharton, V.V., and Bredikhin, S.I., ECS Trans., 2015, vol. 68, p. 1265.
Agarkov, D.A., M. Sc. Dissertation, Chernogolovka: IFTT RAN, 2013.
Burmistrov, I.N., Cand. Sci. (Phys.–Math.) Dissertation, Chernogolovka: IFTT RAN, 2011.
Burmistrov, I., Drozhzhin, O., Istomin, S., Sinitsyn, V., Antipov, E., and Bredikhin, S., J.Electrochem. Soc., 2009, vol. 156, p. B1212.
Burmistrov, I. and Bredikhin, S., ECS Trans., 2009, vol. 25, p. 2793.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.A. Agarkov, I.N. Burmistrov, F.M. Tsybrov, I.I. Tartakovskii, V.V. Kharton, S.I. Bredikhin, 2016, published in Elektrokhimiya, 2016, Vol. 52, No. 7, pp. 673–679.
Published on the basis of the materials of III All-Russia Conference “Fuel Cells and Power Plants on Their Basis,” Chernogolovka, 2015.
Rights and permissions
About this article
Cite this article
Agarkov, D.A., Burmistrov, I.N., Tsybrov, F.M. et al. Kinetics of NiO reduction and morphological changes in composite anodes of solid oxide fuel cells: Estimate using Raman scattering technique. Russ J Electrochem 52, 600–605 (2016). https://doi.org/10.1134/S1023193516070028
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516070028