Abstract
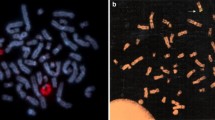
We present results of the study to identify gonosomal mosaicism and examine its inter-tissue variability in peripheral blood lymphocytes, buccal epithelium, and gonadal tissue in patients with disorders of sex development (DSD) associated with sex chromosome abnormalities (gonosomal DSD, n = 8) and gonad differentiation anomalies (46,XY DSD, n = 10). The study used a set of methods for analyzing individual cells: karyoty** and interphase FISH. According to the study results, gonosomal mosaicism was detected in 12 (67%) of the 18 examined patients. Cryptic, isolated by gonadal tissue, somatic mosaicism with 45,X cell line was detected in 4/10 46,XY DSD group patients. True inter-tissue mosaicism with two cell lines, one of which 45,X, was detected in 8/8 gonosomal DSD group patients. We propose using a new approach for mosaicism examination and quantifying mosaic cell lines variability. For this purpose, the coefficient of variation (CV) was used, the range of detected values was from 2.3 to 49%. In accordance with the obtained CV values, the inter-tissue variability was estimated as low, moderate or high. The correlation analysis revealed a statistically significant (p < 0.1) inverse correlation between Prader's stage and 45,X cell line percentage in lymphocytes, buccal epithelium, and gonadal tissue, as well as a direct correlation between the 45,X cell line presence in one of the tissues and its presence in other tissues. The search for mosaicism in tissues of different histogenesis origin using a set of methods for analyzing individual cells allows us to get a more complete picture of the gonosomal constitution and reveal a hidden imbalance, which is very important in examining patients with DSD. We proposed and used in this study the coefficient of variation (CV), which is a convenient statistical metric for quantifying the variability of cell lines in different tissues of a single patient and a step towards standardizing the results of genetic studies.


Similar content being viewed by others
REFERENCES
Hughes, I.A., Houk, C., Ahmed, S.F., et al., Consensus statement on management of intersex disorders, Arch. Dis. Child., 2006, vol. 91, no. 7, pp. 554—563. https://doi.org/10.1136/adc.2006.098319
Gardner, R.J.M. and David, J.A., Chromosome Abnormalities and Genetic Counseling, Oxford Univ. Press, 2018, 5th ed.
Kurilo, L.F., Chromosomal diseases of the reproductive system, Klin. Eksp. Morfol., 2015, no. 1, pp. 48—59.
Kurilo, L.F., Andreeva, M.V., and Kolomiets, O.L., Genetic syndromes with developmental disorders of the reproductive system, Androl. Genital. Khir., 2013, no. 4, pp. 17—27. https://doi.org/10.17650/2070-9781-2013-4-17-27
Rubtsov, N.B., Metody raboty s khromosomami mlekopitayushchikh: uchebnoe posobie (Methods for Working with Mammalian Chromosomes: a Tutorial), Novosibirsk: Novosibirsk. Gos. Univ., 2006.
Kuznetsova, T.V., Shilova, N.V., Tvorogova, M.G., et al., Practical recommendations to ensure quality and safety of cytogenetic research, Med. Genet., 2019, vol. 18, no. 5, pp. 3—27. https://doi.org/10.25557/2073-7998.2019.05.3-27
McGowan-Jordan, J., Simons, A., and Schmid, M., ISCN 2016: an International System for Human Cytogenomic Nomenclature, Cytogenet. Genome Res., 2016, vol. 149, nos. 1—2, pp. 1—140.
R Core Team, R: a language and environment for statistical computing. https://www.R-project.org/. Accessed December 2, 2020.
Vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan. Accessed December 2, 2020.
pwr: Basic Functions for Power Analysis. R package version 1.3-0. https://CRAN.R-project.org/package=pwr. Accessed December 2, 2020.
Efimova, M.R., Petrova, E.V., Ganchenko, O.I., and Mikhailov, M.F., Obshchaya teoriya statistiki: praktikum (General Theory of Statistics: Laboratory Manual), Moscow: Yurait, 2019, 4th ed.
Pisareva, O.M., Metody prognozirovaniya razvitiya sotsial’no-ekonomicheskikh system (Methods for Predicting the Development of Socio-Economic Systems), Moscow: Vysshaya Shkola, 2007.
Gomes, N.L., Chetty, T., Jorgensen, A., and Mitchell, R.T., Disorders of sex development—novel regulators, impacts on fertility, and options for fertility preservation, Int. J. Mol. Sci., 2020, vol. 21, no. 7, p. 2282. https://doi.org/10.3390/ijms21072282
Veitia, R.A., Salas-Cortés, L., Ottolenghi, C., et al., Testis determination in mammals: more questions than answers, Mol. Cell. Endocrinol., 2001, vol. 179, nos. 1–2, pp. 3—16. https://doi.org/10.1016/s0303-7207(01)00460-9
Röpke, A., Kalinski, T., Mohnike, K., et al., Distribution of sex chromosomes in dysgenetic gonads of mixed type, Cytogenet. Genome Res., 2007, vol. 116. nos. 1—2, pp. 146—151. https://doi.org/10.1159/000097435
Röpke, A., Pelz, A.F., Volleth, M., et al., Sex chromosomal mosaicism in the gonads of patients with gonadal dysgenesis, but normal female or male karyotypes in lymphocytes, Am. J. Obstet. Gynecol., 2004, vol. 190, no. 4, pp. 1059—1062. https://doi.org/10.1016/j.ajog.2003.09.053
Berberoğlu, M. and Şiklar, Z., The evaluation of cases with Y-chromosome gonadal dysgenesis: clinical experience over 18 years, J. Clin. Res. Pediatr. Endocrinol., 2018, vol. 10, no. 1, pp. 30—37. https://doi.org/10.4274/jcrpe.4826
Andrade, J.G., Guerra-Júnior, G., and Maciel-Guerra, A.T., 46,XY and 45,X/46,XY testicular dysgenesis: similar gonadal and genital phenotype, different prognosis, Arq. Bras. Endocrinol. Metabol., 2010, vol. 54, no. 3, pp. 331—334. https://doi.org/10.1590/s0004-27302010000300013
Chand, M.T., Turner, S., Solomon, L.A., et al., A case of 45,X/46,XY mosaicism presenting as Swyer syndrome, J. Pediatr. Adolesc. Gynecol., 2020, vol. 33, no. 5, pp. 575—580. https://doi.org/10.1016/j.jpag.2020.06.008
Kamel, A.K., Abd El-Ghany, H.M., Mekkawy, M.K., et al., Sex chromosome mosaicism in the gonads of DSD patients: a karyotype/phenotype correlation, Sex. Dev., 2015, vol. 9, no. 5, pp. 279—288. https://doi.org/10.1159/000442332
Guo, X., Dai, X., Zhou, T., et al., Mosaic loss of human Y chromosome: what, how and why, Hum. Genet., 2020, vol. 139, no. 4, pp. 421—446. https://doi.org/10.1007/s00439-020-02114-w
Faure-Conter, C., Orbach, D., Fresneau, B., et al., Disorder of sex development with germ cell tumors: which is uncovered first?, Pediatr. Blood Cancer, 2020, vol. 67, no. 4. e28169. https://doi.org/10.1002/pbc.28169
Mekkawy, M.K., Kamel, A.K., Dessouky, N., et al., Cytogenetic spectrum of ovotesticular difference of sex development (OT DSD) among a large cohort of DSD patients and literature review, Sex. Dev., 2019, vol. 13, nos. 5—6, pp. 221—227. https://doi.org/10.1159/000508153
Peek, R., Schleedoorn, M., Smeets, D., et al., Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45,X granulosa cells, Hum. Reprod., 2019, vol. 34, no. 9, pp. 1686—1696. https://doi.org/10.1093/humrep/dez135
Reddy, K.S. and Sulcova, V., Pathogenetics of 45,X/46,XY gonadal mosaicism, Cytogenet. Cell Genet., 1998, vol. 82, nos. 1—2, pp. 52—57. https://doi.org/10.1159/000015064
Nomura, R., Miyai, K., Okada, M., et al., A 45,X/46,XY DSD (Disorder of Sexual Development) case with an extremely uneven distribution of 46,XY cells between lymphocytes and gonads, Clin. Pediatr. Endocrinol., 2015, vol. 24, no. 1, pp. 11—14. https://doi.org/10.1297/cpe.24.11
Graff, A., Donadille, B., Morel, H., et al., Added value of buccal cell FISH analysis in the diagnosis and management of Turner syndrome, Hum. Reprod., 2020, vol. 35, no. 10, pp. 2391—2398. https://doi.org/10.1093/humrep/deaa197
Baer, T.G., Freeman, C.E., Cujar, C., et al., Prevalence and physical distribution of SRY in the gonads of a woman with Turner syndrome: phenotypic presentation, tubal formation, and malignancy risk, Horm. Res. Paediatr., 2017, vol. 88, nos. 3—4, pp. 291—297. https://doi.org/10.1159/000477240
Machiela, M.J., Zhou, W., Karlins, E., et al., Female chromosome X mosaicism is age-related and preferentially affects the inactivated X chromosome, Nat. Commun., 2016, no. 7, p. 11843. https://doi.org/10.1038/ncomms11843
Pan, L., Su, Z., Song, J., et al., Growth data and tumour risk of 32 Chinese children and adolescents with 45,X/46,XY mosaicism, BMC Pediatrics, 2019, vol. 19, no. 1, p. 143. https://doi.org/10.1186/s12887-019-1520-9
Chernykh, V.B., Yamandi, T.A., and Safina, N.Yu., New molecular technologies in genetic diagnosis of male infertility, Androl. Genital. Khir., 2017, vol. 18, no. 1, pp. 10—22. https://doi.org/10.17650/2070-9781-2017-18-1-10-22
Funding
This work was carried out without attracting additional funding from third parties.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the preparation of the article and read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement of compliance with standards of research involving humans as subjects. All procedures carried out in a study with the participation of people comply with the ethical standards of the institutional and/or national research ethics committee and the 1964 Helsinki Declaration and its subsequent changes or comparable standards of ethics.
Informed voluntary consent was obtained from each of the participants in the study.
Additional information
Translated by A.A. Kashevarova
Rights and permissions
About this article
Cite this article
Oparina, N.V., Raygorodskaya, N.Y., Latyshev, O.Y. et al. Inter-Tissue Gonosomal Mosaicism in Patients with Disorders of Sex Development, Associated with Abnormalities of Gonadal Differentiation. Russ J Genet 57, 1312–1321 (2021). https://doi.org/10.1134/S1022795421110107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795421110107