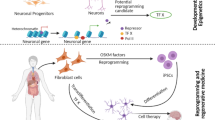
Abstract
Gene function disclosure and the development of modern technologies of genetic manipulations offered the possibility of genetic reprogramming application to alter cell specialization. With the involvement of a gene set that encodes the transcription factors responsible for the pluripotent state, any cell of an adult body could be reprogrammed into the embryonal state and pluripotency could be induced in this cell. Such reprogrammed cells were called induced pluripotent stem cells (iPSCs), and they are capable of again passing through all developmental stages. This provides new possibilities for studies of the basic mechanisms of developmental biology, the formation of specific cell types, and the whole body. In culture, iPSCs could be maintained permanently in a nontransformed state and permit genetic manipulations while maintaining their pluripotent properties. Such a unique combination of their properties makes them an attractive tool for studies of various pathologies and for the delineation of treatment approaches. This review discusses the basic and applied aspects of iPSCs biology.
Similar content being viewed by others
References
Waddington, C.H., The Strategy of the Genes, London: Geo Allen and Unwin, 1957.
Takahashi, K. and Yamanaka, S., Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 2006, no. 126, pp. 663–676.
Evans, M.J. and Kaufman, M.H., Establishment in culture of pluripotential cells from mouse embryos, Nature, 1981, no. 292, pp. 154–156.
Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., et al., Embryonic stem cell lines derived from human blastocysts, Science, 1998, no. 282, pp. 1145–1147.
Hanna, J., Markoulaki, S., Schorderet, P., et al., Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency, Cell, 2008, no. 133, pp. 250–264.
Kim, J.B., Zaehres, H., Wu, G., et al., Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors, Nature, 2008, no. 454, pp. 646–650.
Lagarkova, M.A., Shutova, M.V., Bogomazova, A.N., et al., Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale, Cell Cycle, 2010, no. 9, pp. 937–946.
Liao, J., Cui, C., Chen, S., et al., Generation of induced pluripotent stem cell lines from adult rat cells, Cell Stem Cell, 2009, vol. 9, no. 4(1), pp. 11–15.
Wu, Z., Chen, J., Ren, J., et al., Generation of pig induced pluripotent stem cells with a drug-inducible system, J. Mol. Cell Biol., 2009, no. 1(1), pp. 46–54.
Nakagawa, M., Koyanagi, M., Tanabe, K., et al., Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts, Nat. Biotechnol., 2008, no. 26(1), pp. 101–106.
Banito, A., Rashid, S.T., Acosta, J.C., et al., Senescence impairs successful reprogramming to pluripotent stem cells, Genes Dev., 2009, no. 23(18), pp. 2134–2139.
Shutova, M.V., Bogomazova, A.N., Lagarkova, M.A., and Kiselev, S.L., Generation and characterization of human induced pluripotent stem cells, Acta Nat., 2009, no. 1(2), pp. 91–92.
Shutova, M.V., Chestkov, I.V., Bogomazova, A.N., et al., Generation of iPS cells from human umbilical vein endothelial cells by lentiviral transduction and their differentiation to neuronal lineage, in Human Embryonic and Induced Pluripotent Stem Cells: Lineage-Specific Differentiation Protocols, Springer Protocols Handbooks, 2011, pp. 134–150.
Kim, D., Kim, C.H., Moon, J.I., et al., Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins, Cell Stem Cell, 2009, vol. 5, no. 4(6), pp. 472–476.
Zhou, H., Wu, S., Joo, J.Y., et al., Generation of induced pluripotent stem cells using recombinant proteins, Cell Stem Cell, 2009, vol. 8, no. 4(5), pp. 381–384.
Fusaki, N., Ban, H., Nishiyama, A., et al., Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome, Proc. Jpn. Acad., Ser. B, 2009, vol. 85, no. 8, pp. 348–362.
Takeda, A., Igarashi, H., Kawada, M., et al., Evaluation of the immunogenicity of replication-competent V-knocked-out and replication-defective F-deleted Sendai virus vector-based vaccines in macaques, Vaccine, 2008, vol. 9, no. 26(52), pp. 6839–6843.
Yonemitsu, Y., Kitson, C., Ferrari, S., et al., Efficient gene transfer to airway epithelium using recombinant Sendai virus, Nat. Biotechnol., 2000, no. 18(9), pp. 970–973.
Angel, M., and Yanik, M.F., Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins, PLoS One, 2010, vol. 23, no. 5(7). e11756
Warren, L., Manos, P.D., Ahfeldt, T., et al., Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA, Cell Stem Cell, 2010, vol. 5, no. 7(5), pp. 618–630.
Douvaras, P., Wang, J., Zimmer, M., et al., Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells, Stem Cell Rep., 2014, vol. 12, no. 3(2), pp. 250–259.
Yoshioka, N., Gros, E., Li, H.R., et al., Efficient generation of human iPSCs by a synthetic self-replicative RNA, Cell Stem Cell, 2013, vol. 1, no. 13(2), pp. 246–254.
Zhou, Q., Brown, J., Kanarek, A., et al., In vivo reprogramming of adult pancreatic exocrine cells to betacells, Nature, 2008, vol. 2, no. 455(7213), pp. 627–632.
Su, Z., Niu, W., Liu, M.L., et al., In vivo conversion of astrocytes to neurons in the injured adult spinal cord, Nat. Commun., 2014, vol. 25, no. 5, p. 3338.
Lund, R.J., Närvä, E., and Lahesmaa, R., Genetic and epigenetic stability of human pluripotent stem cells, Nat. Rev. Genet., 2012, no. 13, pp. 732–744.
Karamysheva, T.V., Prokhorovich, M.A., Lagarkova, M.A., et al., Chromosome rearrangements in sublines of human embryonic stem cell lines hESM01 and hESM03, BioDiscovery, 2013, no. 7, p. 1
International Stem Cell Initiative, Amps, K., Andrews, P.W., et al., Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage, Nat. Biotechnol., 2011, no. 29, pp. 1132–1144.
Hussein, S.M.I., Elbaz, J., and Nagy, A., Genome damage in induced pluripotent stem cells: assessing the mechanisms and their consequences, BioEssays, 2012, no. 35(3), pp. 152–162.
Ramos-Mejia, V., Muñoz-Lopez, M., Garcia-Perez, J.L., and Menendez, P., iPSC lines that do not silence the expression of the ectopic reprogramming factors may display enhanced propensity to genomic instability, Cell Res., 2010, no. 20, pp. 1092–1095.
Hussein, S.M., Batada, N.N., Vuoristo, S., et al., Copy number variation and selection during reprogramming to pluripotency, Nature, 2011, no. 471(7336), pp. 58–62.
Bershteyn, M., Hayashi, Y., Desachy, G., et al., Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells, Nature, 2014, no. 507(7490), pp. 99–103.
Lister, R., Pelizzola, M., Kida, Y.S., et al., Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells, Nature, 2011, vol. 3, no. 471(7336), pp. 68–73.
Bock, C., Kiskinis, E., Verstappen, G., et al., Reference maps of human ES and iPS cell variation enable highthroughput characterization of pluripotent cell lines, Cell, 2011, vol. 4, no. 144(3), pp. 439–452.
Nazor, K.L., Altun, G., Lynch, C., et al., Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives, Cell Stem Cell, 2012, vol. 4, no. 10(5), pp. 620–634.
Maherali, N., Sridharan, R., **e, W., et al., Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution, Cell Stem Cell, 2007, vol. 1, pp. 55–70.
Tchieu, J., Kuoy, E., Chin, M.H., et al., Female human iPSCs retain an inactive X chromosome, Cell Stem Cell, 2010, vol. 7, no. (3), pp. 329–342.
Lagarkova, M.A., Shutova, M.V., Bogomazova, A.N., et al., Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale, Cell Cycle, 2010, vol. 1, no. 9(5), pp. 937–946.
Bruck, T. and Benvenisty, N., Meta-analysis of the heterogeneity of X chromosome inactivation in human pluripotent stem cells, Stem Cell Res., 2011, vol. 6, no. 2, pp. 187–193.
Heard, E. and Disteche, C.M., Dosage compensation in mammals: fine-tuning the expression of the X chromosome, Genes Dev., 2006, vol. 20, no. 14, pp. 1848–1867.
Panova, A.V., Nekrasov, E.D., Lagar’kova, M.A., et al., Late replication of the inactive X chromosome is independent of the compactness of chromosome territory in human pluripotent stem cells, Acta Nat., 2013, vol. 5, no. 2(17), pp. 55–63.
Bogomazova, A.N., Lagarkova, M.A., Panova, A.V., et al., Reactivation of X chromosome upon reprogramming leads to changes in the replication pattern and 5hmC accumulation, Chromosoma, 2014, vol. 123, nos. 1–2, pp. 117–128.
Kim, K., Zhao, R., Doi, A., et al., Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells, Nat. Biotechnol., 2011, no. 29, pp. 1117–1119.
Choi, K.D., Vodyanik, M.A., and Slukvin, I.I., Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cellderived lin-CD34+CD43+CD45+ progenitors, J. Clin. Invest., 2009, no. 119, pp. 2818–2829.
Feng, Q., Lu, S.J., Klimanskaya, I., et al., Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence, Stem Cells, 2010, no. 28(4), pp. 704–712.
Ramos-Mejia, V., Melen, G.J., Sanchez, L., et al., Nodal/Activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage, Mol. Ther., 2010, no. 18, pp. 2173–2181.
Qiu, C., Olivier, E.N., Velho, M., and Bouhassira, E.E., Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells, Blood, 2008, no. 111, pp. 2400–2408.
Chang, C.J., Mitra, K., Koya, M., et al., Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells, PLoS One, 2011, no. 6. e25761
Lagarkova, M.A., Eremeev, A.V., Svetlakov, A.V., et al., Human embryonic stem cell lines isolation, cultivation, and characterization, In Vitro Cell Dev. Biol. Anim., 2010, no. 46(3–4), pp. 284–293.
Klimanskaya, I., Hipp, J., Rezai, K.A., et al., Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics, Cloning Stem Cells, 2004, no. 6(3), pp. 217–245.
Lamba, D.A., Gust, J., and Reh, T.A., Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice, Cell Stem Cell, 2009, no. 4(1), pp. 73–79.
Maeda, T., Lee, M.J., Palczewska, G., et al., Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo, J. Biol. Chem., 2013, no. 288(48), pp. 34484–34493.
Meyer, J.S., Howden, S.E., Wallace, K.A., et al., Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment, Stem Cells, 2011, no. 29(8), pp. 1206–12018.
Lagar’kova, M.A., Shilov, A.G., Gubanova, N.I., et al., Histogenesis of human embryonic stem cells in vitro into the retinal components, Kletochnye Tekhnol. Biol. Med., 2011, no. 4, pp. 203–205.
Phillips, M.J., Perez, E.T., Martin, J.M., et al., Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2, Stem Cells, 2014, no. 32(6), pp. 1480–1492.
Yoshida, T., Ozawa, Y., Suzuki, K., et al., The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa, Mol. Brain, 2014, vol. 16, no. 7, p. 45.
Schwartz, S.D., Hubschman, J.P., Heilwell, G., et al., Embryonic stem cell trials for macular degeneration: a preliminary report, Lancet, 2012, vol. 25, no. 379(9817), pp. 713–720.
Kamao, H., Mandai, M., Okamoto, S., et al., Characterization of human induced pluripotent stem cellderived retinal pigment epithelium cell sheets aiming for clinical application, Stem Cell Rep., 2014, no. 2(2), pp. 205–218.
Park, I.-H., Arora, N., Huo, H., et al., Disease-specific induced pluripotent stem cells, Cell, 2008, no. 134, pp. 877–886.
Brennand, K.J., Simone, A., Jou, J., et al., Modelling schizophrenia using human induced pluripotent stem cells, Nature, 2011, no. 473, pp. 221–225.
Israel, M.A., Yuan, S.H., Bardy, C., et al., Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells, Nature, 2012, no. 482, pp. 216–220.
Kondo, T., Asai, M., Tsukita, K., et al., Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness, Cell Stem Cell, 2013, no. 12, pp. 487–496.
Nguyen, H.N., Byers, B., Cord, B., et al., LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress, Cell Stem Cell, 2011, no. 8, pp. 267–280.
Liu, G.-H., Qu, J., Suzuki, K., et al., Progressive degeneration of human neural stem cells caused by pathogenic LRRK2, Nature, 2012, no. 491, pp. 603–607.
Chung, C.Y., Khurana, V., Auluck, P.K., et al., Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons, Science, 2013, no. 342, pp. 983–987.
The HD iPSC Consortium, Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes, Cell Stem Cell, 2012, no. 11, pp. 264–278.
Nekrasov, E.D., Lebedeva, O.S., Chestkov, I.V., et al., Generation and characterization of induced pluripotent stem cells from human skin fibroblasts of patients with neurodegenerative diseases, Kletochnaya Transplantol. Tkanevaya Inzheneriya, 2011, no. 6, pp. 82–88.
Nekrasov, E.D., Lebedeva, O.S., Vasina, E.M., et al., A platform for studies of Huntington’s disease on the basis of induced pluripotent stem cells, Ann. Klin. Eksp. Nevrol., 2012, no. 6, pp. 30–35.
Chestkov, I.V., Vasilieva, E.A., Illarioshkin, S.N., et al., Patient-specific induced pluripotent stem cells for SOD1-associated amyotrophic lateral sclerosis pathogenesis studies, Acta Nat., 2014, no. 6, pp. 54–60.
Lebedeva, O.S., Lagar’kova, M.A., Kiselev, S.L., et al., The morphofunctional properties of induced pluripotent stem cells derived from human skin fibroblasts and differentiated to dopaminergic neurons, Neurochem. J., 2013, vol. 7, no. 3, pp. 207–214.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Bogomazova, E.M. Vassina, S.L. Kiselev, M.A. Lagarkova, O.S. Lebedeva, E.D. Nekrasov, A.V. Panova, E.S. Philonenko, E.A. Khomyakova, L.V. Tskhovrebova, I.V. Chestkov, M.V. Shutova, 2015, published in Genetika, 2015, Vol. 51, No. 4, pp. 466–478.
All of the authors equally contributed to the writing of this paper.
Rights and permissions
About this article
Cite this article
Bogomazova, A.N., Vassina, E.M., Kiselev, S.L. et al. Genetic cell reprogramming: A new technology for basic research and applied usage. Russ J Genet 51, 386–396 (2015). https://doi.org/10.1134/S102279541504002X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102279541504002X