Abstract
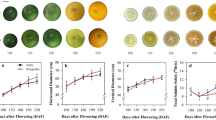
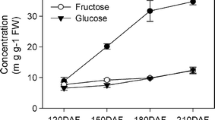
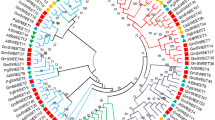
Tomato (Solanum lycopersicum L.) is an important crop and, due to the existence of wild related species (Solanum section Lycopersicon), a model for studying the development of the fleshy fruit. In the study, expression analysis of sugar uniporter genes SWEET1а, 1b, 1e, 3, 7a, 10a, 12c, 14, and 15 in tomato species and cultivars was carried out. In cv. Heinz (S. lycopersicum), genes that are most active in roots (SWEET1e, 3, 10a, and 12c), leaves (SWEET1a, 1e, 3, 10a, and 12c) and flowers (SWEET1a, 1b, 7a, 10a, 12s, 14, and 15) were revealed. The growth of the fruit is accompanied by an increase in the level of the SWEET 110a and 12c transcripts; maturation of the fruit is accompanied by an increase in the level of the SWEET 1a and 15 transcripts. Differential expression of the SWEET1a, 1b, 12c, and 15 genes in the ripe fruit of inbred lines obtained from crossing of S. lycopersicum cv. M82 × S. pennellii was demonstrated. qRT-PCR analysis showed that the expression of the SWEET1a and 12c genes is common for ripe fruit of the analyzed tomato species, while the expression of the SWEET1b and 10a genes is common for S. pennellii, S. habrochaites, and S. cheesmaniae. It was determined that the fructose : glucose ratio is equimolar in the accessions except for cv. Black Jack and White Beauty (fructose : glucose ≥ 1.10). Correlations between the level of SWEET gene transcripts and the ratio of hexoses was not revealed.




Similar content being viewed by others
REFERENCES
Yoon, J., Cho, L.H., Tun, W., Jeon, J.S., and An, G., Sucrose signaling in higher plants, Plant Sci., 2021, vol. 302, p. 110703. https://doi.org/10.1016/j.plantsci.2020.110703
Sami, F., Siddiqui, H., and Hayat, S., Interaction of glucose and phytohormone signaling in plants, Plant Physiol. Biochem., 2019, vol. 135, p. 119. https://doi.org/10.1016/j.plaphy.2018.11.005
Ji, Y., Nuñez Ocaña, D., Choe, D., Larsen, D.H., Marcelis, L.F.M., and Heuvelink, E., Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato, New Phytol., 2020, vol. 228, p. 1914. https://doi.org/10.1111/nph.16805
Zhang, Z., Zou, L., Ren, C., Ren, F., Wang, Y., Fan, P., Li, S., and Liang, Z., VvSWEET10 mediates sugar accumulation in grapes, Genes (Basel)., 2019, vol. 10, p. 255. https://doi.org/10.3390/genes10040255
Peralta, I.E. and Spooner, D.M., Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon), Am. J. Bot., 2001, vol. 88, p. 1888.
Davies, J.N., Occurrence of sucrose in the fruit of some species of Lycopersicon, Nature, 1966, vol. 209, p. 640. https://doi.org/10.1038/209640a0
Miron, D. and Schaffer, A.A., Sucrose phosphate synthase, sucrose synthase, and invertase activities in develo** fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl., Plant Physiol., 1991, vol. 95, p. 623. https://doi.org/10.1104/pp.95.2.623
Beckles, D.M., Hong, N., Stamova, L., and Luengwilai, K., Biochemical factors contributing to tomato fruit sugar content: a review, Fruits, 2012, vol. 67, p. 49. https://doi.org/10.1051/fruits/2011066
Shammai, A., Petreikov, M., Yeselson, Y., Faigenboim, A., Moy-Komemi, M., Cohen, S., Cohen, D., Besaulov, E., Efrati, A., Houminer, N., Bar, M., Ast, T., Schuldiner, M., Klemens, P.A.W., Neuhaus, E., et al., Natural genetic variation for expression of a SWEET transporter among wild species of Solanum lycopersicum (tomato) determines the hexose composition of ripening tomato fruit, Plant J., 2018, vol. 96, p. 343. https://doi.org/10.1111/tpj.14035
Levin, I., Gilboa, N., Yeselson, E., Shen, S., and Schaffer, A.A., Fgr, a major locus that modulates fructose to glucose ratio in mature tomato fruit, Theor. Appl. Genet., 2000, vol. 100, p. 256. https://doi.org/10.1007/s001220050034
Ho, L.H., Klemens, P.A.W., Neuhaus, H.E., Ko, H.Y., Hsieh, and S.Y., Guo, W.J., SlSWEET1a is involved in glucose import to young leaves in tomato plants, J. Exp. Bot., 2019, vol. 70, p. 3241. https://doi.org/10.1093/jxb/erz154
Julius, B.T., Leach, K.A., Tran, T.M., Mertz, R.A., and Braun, D.M., Sugar transporters in plants: new insights and discoveries, Plant Cell Physiol., 2017, vol. 58, p. 1442. https://doi.org/10.1093/pcp/pcx090
Chen, L.Q., SWEET sugar transporters for phloem transport and pathogen nutrition, New Phytol., 2014, vol. 201, p. 1150. https://doi.org/10.1111/nph.12445
Chen, L.-Q., Qu, X.-Q., Hou, B.-H., Sosso, D., Osorio, S., Fernie, A.R., and Frommer, W.B., Sucrose efflux mediated by SWEET proteins as a key step for phloem transport, Science, 2012, vol. 335, p. 207. https://doi.org/10.1126/science.1213351
Patil, G., Valliyodan, B., Deshmukh, R., Prince, S., Nicander, B., Zhao, M., Sonah, H., Song, L., Lin, L., Chaudhary, J., Liu, Y., Joshi, T., Xu, D., and Ngu-yen, H.T., Soybean (Glycine max) SWEET gene family: insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis, BMC Genomics, 2015, vol. 16, p. 520. https://doi.org/10.1186/s12864-015-1730-y
Klemens, P.A., Patzke, K., Deitmer, J., Spinner, L., Le, Hir R., Bellini, C., Bedu, M., Chardon, F., Krapp, A., and Neuhaus, H.E., Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis, Plant Physiol., 2013, vol. 163, p. 1338. https://doi.org/10.1104/pp.113.224972
Chen, L.Q., Hou, B.H., Lalonde, S., Takanaga, H., Hartung, M.L., Qu, X.Q., Guo, W.J., Kim, J.G., Underwood, W., Chaudhuri, B., Chermak, D., Antony, G., and White, F.F., Somerville S.C., Mudgett, M.B., et al., Sugar transporters for intercellular exchange and nutrition of pathogens, Nature, 2010, vol. 468, p. 527. https://doi.org/10.1038/nature09606
Eom, J.S., Chen, L.Q., Sosso, D., Julius, B.T., Lin, I.W., Qu, X.Q., Braun, D.M., and Frommer, W.B., SWEETs, transporters for intracellular and intercellular sugar translocation, Curr. Opin. Plant Biol., 2015, vol. 25, p. 53. https://doi.org/10.1016/j.pbi.2015.04.005
Isoda, R., Palmai, Z., Yoshinari, A., Chen, L.Q., Tama, F., Frommer, W.B., and Nakamura, M., SWEET13 transport of sucrose, but not gibberellin, restores male fertility in Arabidopsis sweet13; 14, Proc. Natl. Acad. Sci. U.S.A., 2022, vol. 119, p. e2207558119. https://doi.org/10.1073/pnas.2207558119
Chen, H.Y., Huh, J.H., Yu, Y.C., Ho, L.H., Chen, L.Q., Tholl, D., Frommer, W.B., and Guo, W.J., The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection, Plant J., 2015, vol. 83, p. 1046. https://doi.org/10.1111/tpj.12948
Valifard, M., Le Hir, R., Müller, J., Scheuring, D., Neuhaus, H.E., and Pommerrenig, B., Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance, Plant Physiol., 2021, vol. 187, p. 2716. https://doi.org/10.1093/plphys/kiab436
Feng, C.Y., Han, J.X., Han, X.X., and Jiang, J., Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato, Gene, 2015, vol. 573, p. 261. https://doi.org/10.1016/j.gene.2015.07.055
Wang, J., Yu, Y.C., Li, Y., and Chen, L.Q., Hexose transporter SWEET5 confers galactose sensitivity to Arabidopsis pollen germination via a galactokinase, Plant Physiol., 2022, vol. 189, p. 388. https://doi.org/10.1093/plphys/kiac068
Lin, I., Sosso, D., Chen, L.Q., Gase, K., Kim, S.G., Kessler, D., Klinkenberg, P.M., Gorder, M.K., Hou, B.H., Qu, X.Q., Carter, C.J., Baldwin, I.T., and Frommer, W.B., Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9, Nature, 2014, vol. 508, p. 546. https://doi.org/10.1038/nature13082
Chen, L.Q., Lin, I.W., Qu, X.Q., Sosso, D., McF-arlane, H.E., Londoño, A., Samuels, A.L., and Frommer, W.B., A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo, Plant Cell, 2015, vol. 27, p. 607. https://doi.org/10.1105/tpc.114.134585
Zhang, X., Feng, C., Wang, M., Li, T., Liu, X., and Jiang J., Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits, Hortic. Res., 2021, vol. 8, p. 186. https://doi.org/10.1038/s41438-021-00624-w
Ko, H.Y., Ho, L.H., Neuhaus, H.E., and Guo, W.J., Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato, Plant Physiol., 2021, vol. 187, p. 2230. https://doi.org/10.1093/plphys/kiab290
Efremov, G.I., Slugina, M.A., Shchennikova, A.V., and Kochieva, E.Z., Differential regulation of phytoene synthase PSY1 during fruit carotenogenesis in cultivated and wild tomato species (Solanum section Lycopersicon), Plants, 2020, vol. 9, p. 1169. https://doi.org/10.3390/plants9091169
Fortuny, A.P., Bueno, R.A., Pereira da Costa, J.H., Zanor, M.I., and Rodríguez, G.R., Tomato fruit quality traits and metabolite content are affected by reciprocal crosses and heterosis, J. Exp. Bot., 2021, vol. 72, p. 5407. https://doi.org/10.1093/jxb/erab222
Klee, H.J. and Giovannoni, J.J., Genetics and control of tomato fruit ripening and quality attributes, Annu. Rev. Genet., 2011, vol. 45, p. 41. https://doi.org/10.1146/annurev-genet-110410-132507
Jia, H., Jiu, S., Zhang, C., Wang, C., Tariq, P., Liu, Z., Wang, B., Cui, L., and Fang, J., Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor, Plant Biotechnol. J., 2016, vol. 14, p. 2045. https://doi.org/10.1111/pbi.12563
De Rocchis, V., Jammer, A., Camehl, I., Franken, P., and Roitsch, T., Tomato growth promotion by the fungal endophytes Serendipita indica and Serendipita herbamans is associated with sucrose de-novo synthesis in roots and differential local and systemic effects on carbohydrate metabolisms and gene expression, J. Plant Physiol., 2022, vol. 276, p. 153755. https://doi.org/10.1016/j.jplph.2022.153755
Cai, Y., Yin, L., Tu, W., Deng, Z., Yan, J., Dong, W., Gao, H., Xu, J., Zhang, N., Wang, J., Zhu, L., Meng, Q., and Zhang, Y., Ectopic Expression of VvSUC27 Induces Stenospermocarpy and Sugar Accumulation in Tomato Fruits, Front. Plant Sci., 2021, vol. 12, p. 759047. https://doi.org/10.3389/fpls.2021.759047
Eshed, Y., Abu-Abied, M., Saranga, Y., and Zamir, D., Lycopersicon esculentum lines containing small overlap** introgressions from L. pennellii, Theor. Appl. Genet., 1992, vol. 83, p. 1027. https://doi.org/10.1007/BF00232968
Funding
The work was supported by the Russian Science Foundation, grant no. 19‒16‒00016 and the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by V. Mittova
Supplementary Information
Rights and permissions
About this article
Cite this article
Filyushin, M.A., Slugina, M.A., Shchennikova, A.V. et al. Differential Expression of Sugar Uniporter Genes of the SWEET Family in the Regulation of Qualitative Fruit Traits in Tomato Species (Solanum Section Lycopersicon). Russ J Plant Physiol 70, 70 (2023). https://doi.org/10.1134/S102144372360023X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372360023X