Abstract
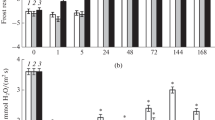
The effect of photoperiod duration on efficiency of low-temperature hardening was investigated in Arabidopsis thaliana (L.) Heynh. plants, ecotype Col-0. Six-week-old plants were exposed to cold acclimation at a temperature of 2°С during 1‒5 days at photoperiods of 0, 8, and 16 h (illuminance of 200 µmol/(m2 s)). According to survival data and leakage of electrolytes after test freezing (–6°C, 24 h), the plants exposed to cold acclimation in the dark did not show frost resistance. The plants hardened in the light (irrespective of the length of photoperiod) considerably improved their frost resistance by the end of the cold-acclimation period. Net photosynthesis/dark respiration ratio in these plants was almost two times greater than in control material (without hardening). The plants exposed to a 16-h-long photoperiod surpassed the type of treatment with 8-h-long illumination both in the highest levels of accumulation of sugars (by almost 40%) and in the rate of reaching these levels in daily dynamics of hardening. It was shown that MDA content transiently rose during the first 24 h of hardening in the light and did not change in the dark, which may point to a signal role of lipid peroxidation products upon cold acclimation. It was discovered that the photoperiod duration affected the formation rate of frost resistance in A. thaliana plants. A more prolonged operation of A. thaliana’s photosynthetic apparatus at 16-h-long photoperiod considerably accelerated the accumulation of sugars upon cold acclimation and, therefore, hastened development of frost resistance as compared with an 8-h-long photoperiod. It was concluded that rapid formation of frost resistance in A. thaliana requires a combination of low above-zero temperature and 16-h-long photoperiod.



Similar content being viewed by others
REFERENCES
Nievola, C.C., Carvalho, C.P., Carvalho, V., and Rodrigues, E., Rapid responses of plants to temperature changes, Temperature, 2017, vol. 4, p. 371. https://doi.org/10.1080/23328940.2017.1377812
Larcher, W., Physiological Plant Ecology. Ecophysiology and Stress Physiology of Functional Groups, Berlin: Springer, 2003, p. 513.
Trunova, T.I., The plant and low temperature stress, 64-e Timiryazevskie chteniya (64th Timiryazev Readings), Moscow: Nauka, 2007.
Theocharis, A., Clement, C., and Barka, E.A., Physiological and molecular changes in plants grown at low temperatures, Planta, 2012, vol. 235, p. 1091. https://doi.org/10.1007/s00425-012-1641-y
Rihan, H.Z., Al-Issawi, M., and Fuller, M.P., Advances in physiological and molecular aspects of plant cold tolerance, J. Plant Interact., 2017, vol. 12, p. 143. https://doi.org/10.1080/17429145.2017.1308568
Weiser, C.J., Cold resistance and injury in woody plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage, Science, 1970, vol. 169, p. 1269. https://doi.org/10.1126/science.169.3952.1269
Maurya, J.P. and Bhalerao, R.P., Photoperiod and temperature mediated control of growth cessation and dormancy in trees: a molecular perspective, Ann. Bot., 2017, vol. 120, p. 351. https://doi.org/10.1093/aob/mcx061
Wanner, L.A. and Junttila, O., Cold-Induced Freezing Tolerance in Arabidopsis, Plant Physiology, 1999, vol. 120, p. 391. https://doi.org/10.1104/pp.120.2.391
Lee, C.M. and Thomashow, M.F., Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana, PNAS, 2012, vol. 109, p. 15054. https://doi.org/10.1073/pnas.1211295109
Trunova, T.I., Light and temperature conditions during slaughtering winter wheat and the importance of oligosaccharides for frost resistance, Fiziol. Rast., 1965, vol. 12, p. 85.
**n, Z. and Browse, J., Cold comfort farm: the acclimation of plants to freezing temperatures, Plant Cell Environ., 2000, vol. 23, p. 893. https://doi.org/10.1046/j.1365-3040.2000.00611.x
Campos, P.S., Quartin, V., Ramalho, J.C., and Nunes, M.A., Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants, J. Plant Physiol., 2003, vol. 160, p. 283. https://doi.org/10.1078/0176-1617-00833
Klimov, S.V., Astakhova, N.V., and Trunova, T.I., Changes in photosynthesis, dark respiration rates and photosynthetic carbon partitioning in winter rye and wheat seedlings during cold hardening, J. Plant Physiol., 1999, vol. 155, p. 734.
Turkina, M.V. and Sokolova, S.V., Methods for the determination of monosaccharides and oligosaccharides, Biokhimicheskie metody v fiziologii rasteniy (Biochemical Methods in Plant Physiology), Pavlinova, O.A., Ed., Moscow: Nauka, 1971.
Heath, R.L. and Packer, L., Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation, Arch Biochem. Biophys., 1968, vol. 125, p. 189. https://doi.org/10.1016/0003-9861(68)90654-1
Zuther, E., Schulz, E., Childs, L.H., and Hincha, D.K., Clinal variation in the non-acclimated and cold–acclimated freezing tolerance of Arabidopsis thaliana accessions, Plant Cell Environ., 2012, vol. 35, p. 1860. https://doi.org/10.1111/j.1365-3040.2012.02522.x
Catala, R., Medina, J., and Salinas, J., Integration of low temperature and light signaling during cold acclimation response in Arabidopsis, PNAS, 2011, vol. 108, p. 16475. https://doi.org/10.1073/pnas.1107161108
Ashworth, E.N. and Pearce, R.S., Extracellular freezing in leaves of freezing-sensitive species, Planta, 2002, vol. 214, p. 798. https://doi.org/10.1007/s00425-001-0683-3
Reyes-Diaz, M., Ulloa, N., Zuniga-Feest, A., Gutierrez, A., Gidekel, M., Alberdi, M., Corcuera, L.J., and Bravo, L.A., Arabidopsis thaliana avoids freezing by supercooling, J. Exp. Bot., 2006, vol. 57, p. 3687. https://doi.org/10.1093/jxb/erl125
Deryabin, A.N. and Trunova, T.I., Colligative effects of solutions of low-molecular sugars and their role in plants under hypothermia, Biol. Bull. Russ. Acad. Sci., 2021, vol. 48, p. 29. https://doi.org/10.1134/S1062359021060042
Kreslavski, V.D., Los, D.A., Allakhverdiev, S.I., and Kuznetsov, V.V., Signaling role of reactive oxygen species in plants under stress, Russ. J. Plant Physiol., 2012, vol. 59, p. 141. https://doi.org/10.1134/S1021443712020057
Foyer, C.H. and Noctor, G., Redox regulation in photosynthetic organisms: signaling, acclimation and practical implications, Antioxid. Redox Signal., 2009, vol. 11, p. 861. https://doi.org/10.1089/ars.2008.2177
Shulaev, V. and Oliver, D.J., Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research, Plant Physiol., 2006, vol. 141, p. 367. https://doi.org/10.1104/pp.106.077925
Mori, I.C. and Schroeder, J.I., Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction, Plant Physiol., 2004, vol. 135, p. 702. https://doi.org/10.1104/pp.104.042069
Pitzschke, P. and Hirt, H., Mitogen activated protein kinases and reactive oxygen species signaling in plants, Plant Physiol., 2006, vol. 141, p. 351. https://doi.org/10.1104/pp.106.079160
Foyer, C.H. and Noctor, G., Redox homeostis and antioxidant signaling: a metabolic interface between stress perception and physiological responses, Plant Cell, 2005, vol. 17, p. 1866. https://doi.org/10.1105/tpc.105.033589
Soitamo, A.J., Piippo, M., Allahverdiyeva, Y., Battchikova, N., and Aro, E.M., Light has a specific role in modulating Arabidopsis gene expression at low temperature, BMC Plant Biology, 2008, vol. 8, p. 13. https://doi.org/10.1186/1471-2229-8-13
Kim, H.J., Kim, Y.K., Park, J.Y., and Kim, J., Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana, Plant J., 2002, vol. 29, p. 693. https://doi.org/10.1046/j.1365-313x.2002.01249.x
Crosatti, C., Polverino de Laureto, P., Bassi, R., and Cattivelli, L., The interaction between cold and light controls the expression of the cold-regulated barley gene cor14b and the accumulation of the corresponding protein, Plant Physiol., 1999, vol. 119, p. 671. https://doi.org/10.1104/pp.119.2.671
Zhang, R., Gonze, D., Hou, X., You, X., and Goldbeter, A., A computational model for the cold response pathway in plants, Front. Physiol., 2020, vol. 11, p. e591073. https://doi.org/10.3389/fphys.2020.591073
Zhao, C., Zhang, Z., **e, S., Si, T., Li, Y., and Zhu, J.K., Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis, Plant Physiol., 2016, vol. 171, p. 2744. https://doi.org/10.1104/pp.16.00533
Funding
This work was performed within the framework of a state assignment given by the Ministry of Science and Higher Education of the Russian Federation (project no. 122042700044-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans and animals as subjects. The authors declare that they have no conflicts of interest.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Balakshina
Abbreviations: (ETC) electron transport chain, (LPO) lipid peroxidation, (MDA) malonic dialdehyde.
Rights and permissions
About this article
Cite this article
Popov, V.N., Deryabin, A.N. Effect of Photoperiod Duration on Efficiency of Low-Temperature Hardening of Arabidopsis thaliana Heynh. (L.). Russ J Plant Physiol 70, 56 (2023). https://doi.org/10.1134/S1021443722603093
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722603093