Abstract
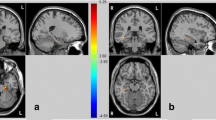
Schizotypy, a set of behavioral traits related to an enhanced risk for mental disorders, is an informative model for the investigation of early predisposition markers. We aimed to analyze correlations between the characteristics of functional brain organization and schizotypy in mentally healthy individuals. Mentally healthy participants (N = 80 in the main sample and N = 32 in the replication sample) underwent resting-state fMRI and Schizotypal Personality Questionnaire (SPQ-74). Correlations between functional connectivity (FC) within and between the brain neuronal networks and four factors of schizotypy were analyzed. Additionally, we tested the whole-brain FC of the right medial frontal cortex for correlations with schizotypy (the region of interest was chosen on the basis of multicenter study of schizotypy associations with brain anatomy). Statistically significant results were tested in the replication sample. The FC within the ventral attention/salience network correlated with negative schizotypy, whilst the FC within the default mode network was associated with disorganization schizotypy (the results did not survive the correction for multiple analyses). Lower FC between the right medial frontal cortex and a temporal-occipital region in the left hemisphere correlated with higher cognitive-perceptual schizotypy, which may reflect deviations in emotional and motivational mediation of visual perception. These results, however, were not replicated in the second sample. Further research in this direction should engage larger samples and take into account a wider spectrum of parameters (psychometric, neuropsychological, and neuroimaging).


Similar content being viewed by others
Notes
This sample was not identical to but substantially overlapped with a sample of 81 subjects whose resting-state fMRI data were obtained earlier with our participation in the project funded by Russian Foundation for Basic Research (RFBR) grant 18-00-01598 (18-00-01592) and are publicly available at https://openneuro.org/datasets/ds003469.
The visual network was not included in the analysis as different instructions were used for the main and replication samples, with open versus closed eyes.
ROI = region of interest.
REFERENCES
Venables, P.H. and Raine, A., The stability of schizotypy across time and instruments, Psychiatry Res., 2015, vol. 228, no. 3, p. 585.
Barrantes-Vidal, N., Grant, P., and Kwapil, T.R., The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders, Schizophr. Bull., 2015, vol. 41, suppl. 2, p. S408.
Alfimova, M.V., Lezheiko, T.V., Sergeev, N.V. et al., Structure of schizotypal traits in the Russian population, Zh. Nevrol. Psikhiatrii im. S. S. Korsakova, 2020, vol. 120, no. 7, p. 94.
Racioppi, A., Sheinbaum, T., Gross, G.M., et al., Prediction of prodromal symptoms and schizophrenia-spectrum personality disorder traits by positive and negative schizotypy: a 3-year prospective study, PLoS One, 2018, vol. 13, no. 11. e0207150
Lenzenweger, M.F., Schizotypy 17 years on: psychotic symptoms in midlife, J. Abnorm. Psychol., 2021, vol. 130, no. 4, p. 399.
Zhao, W., Guo, S., Linli, Z., et al., Functional, anatomical, and morphological networks highlight the role of basal ganglia—thalamus—cortex circuits in schizophrenia, Schizophr. Bull., 2020, vol. 46, no. 2, p. 422.
Waltmann, M., O’Daly, O., Egerton, A., et al., Multi-echo fMRI, resting-state connectivity, and high psychometric schizotypy, NeuroImage Clin., 2019, vol. 21, p. 101603.
Kozhuharova, P., Saviola, F., Diaconescu, A., and Allen, P., High schizotypy traits are associated with reduced hippocampal resting state functional connectivity, Psychiatry Res. Neuroimaging, 2021, vol. 307, p. 111215.
Pettersson-Yeo, W., Allen, P., Benetti, S. et al., Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev., 2011, vol. 35, no. 5, p. 1110.
Wang, Y., Ettinger, U., Meindl, T., and Chan, R.C.K., Association of schizotypy with striatocortical functional connectivity and its asymmetry in healthy adults, Hum. Brain. Mapp., 2018, vol. 39, no. 1, p. 288.
Wang, L.L., Sun, X., Chiu, C.D., et al., Altered cortico-striatal functional connectivity in people with high levels of schizotypy: a longitudinal resting-state study, Asian J. Psychiatr., 2021, vol. 58, p. 102621.
Lagioia, A., Van De Ville, D., Debbane, M. et al., Adolescent resting state networks and their associations with schizotypal trait expression, Front. Syst. Neurosci., 2010, vol. 4., p. 35.
Wang, Y.M., Cai, X.L., Zhang, R.T., et al., Altered brain structural and functional connectivity in schizotypy, Psychol. Med., 2020, vol. 52, no. 5, p. 834.
Wang, Y.M., Cai, X.L., Zhou, H.Y., et al., Altered default mode network functional connectivity in individuals with co-occurrence of schizotypy and obsessive-compulsive traits, Psychiatry Res. Neuroimaging, 2020, vol. 305, p. 111170.
Wang, Y., Yan, C., Yin, D.Z., et al., Neurobiological changes of schizotypy: evidence from both volume-based morphometric analysis and resting-state functional connectivity, Schizophr. Bull., 2015, vol. 41, sup-pl. 2, p. S444.
Kirschner, M., Hodzic-Santor, B., Antoniades, M., et al., Cortical and subcortical neuroanatomical signatures of schizotypy in 3004 individuals assessed in a worldwide ENIGMA study, Mol. Psychiatry, 2021, vol. 27, no. 2, p. 1167.
Raine, A., Reynolds, C., Lencz, T., et al., Cognitive-perceptual, interpersonal, and disorganized features of schizotypal personality, Schizophr. Bull., 1994, vol. 20, no. 1, p. 191.
Efremov, A.G. and Enikolopov, S.N., The approbation of the Cloninger’s biosocial temperament and character inventory (TCI-125) and the schizotypal personality questionnaire (SPQ-74), Vestn. Mosk. Gos. Univ., Ser. 14: Psikhol., 2002, vol. 1, p. 92.
Stefanis, N.C., Smyrnis, N., Avramopoulos, D., et al., Factorial composition of self-rated schizotypal traits among young males undergoing military training, Schizophr. Bull., 2004, vol. 30, no. 2, p. 335.
Hanaie, R., Mohri, I., Kagitani-Shimono, K., et al., Aberrant cerebellar-cerebral functional connectivity in children and adolescents with autism spectrum disorder, Front. Hum. Neurosci., 2018, vol. 12, p. 454.
Yoo, K., Rosenberg, M.D., Hsu, W.T., et al., Connectome-based predictive modeling of attention: comparing different functional connectivity features and prediction methods across datasets, NeuroImage, 2018, vol. 167, p. 11.
Ren, J., Hubbard, C.S., Ahveninen, J., et al., Dissociable auditory cortico-cerebellar pathways in the human brain estimated by intrinsic functional connectivity, Cereb. Cortex, 2021, vol. 31, no. 6, p. 2898.
Yeo, B.T., Krienen, F.M., Sepulcre, J., et al., The organization of the human cerebral cortex estimated by intrinsic functional connectivity, J. Neurophysiol., 2011, vol. 106, no. 3, p. 1125.
Desikan, R.S., Segonne, F., Fischl, B., et al., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest, NeuroImage, 2006, vol. 31, no. 3, p. 968.
Grant, P., Is schizotypy per se a suitable endophenotype of schizophrenia? Do not forget to distinguish positive from negative facets, Front. Psychiatry., 2015, vol. 6, p. 143.
Spechler, P.A., Chaarani, B., Orr, C., et al., Neuroimaging evidence for right orbitofrontal cortex differences in adolescents with emotional and behavioral dysregulation, J. Am. Acad. Child Adolesc. Psychiatry, 2019, vol. 58, no. 11, p. 1092.
Nejati, V., Salehinejad, M.A., and Nitsche, M.A., Interaction of the left dorsolateral prefrontal cortex (l-DLPFC) and right orbitofrontal cortex (OFC) in hot and cold executive functions: evidence from transcranial direct current stimulation (tDCS), Neuroscience, 2018, vol. 369, p. 109.
Rudebeck, P.H. and Rich, E.L., Orbitofrontal cortex, Curr. Biol., 2018, vol. 28, no. 18, p. R1083.
Conway, B.R., The organization and operation of inferior temporal cortex, Annu. Rev. Vis. Sci., 2018, vol. 4, p. 381.
Weiner, K.S., Zilles, K., The anatomical and functional specialization of the fusiform gyrus, Neuropsychologia, 2016, vol. 83, p. 48.
Zeng, H., Fink, G.R., Weidner, R., Visual size processing in early visual cortex follows lateral occipital cortex involvement, J. Neurosci., 2020, vol. 40, no. 22, p. 4410.
Cicero, D.C. and Kerns, J.G., Can disorganized and positive schizotypy be discriminated from dissociation? J. Pers., 2010, vol. 78, no. 4, p. 1239.
Smallwood, J., Bernhardt, B. C., Leech, R., et al., The default mode network in cognition: a topographical perspective, Nat. Rev. Neurosci., 2021, vol. 22, no. 8, p. 503.
Kwapil, T.R., Brown, L.H., Silvia, P.J., et al., The expression of positive and negative schizotypy in daily life: an experience sampling study, Psychol. Med., 2012, vol. 42, no. 12, p. 2555.
Uddin, L.Q., Salience processing and insular cortical function and dysfunction, Nat. Rev. Neurosci., 2015, vol. 16, no. 1, p. 55.
Rosen, M.L., Sheridan, M.A., Sambrook, K.A., et al., Salience network response to changes in emotional expressions of others is heightened during early adolescence: relevance for social functioning, Dev. Sci., 2018, vol. 21, no. 3, p. e12571.
Pisoni, A., Davis, S.W., and Smoski, M., Neural signatures of saliency-map** in anhedonia: a narrative review, Psychiatry Res., 2021, vol. 304, p. 114123.
Limongi, R., Jeon, P., Mackinley, M., et al., Glutamate and disconnection in the salience network: neurochemical, effective connectivity, and computational evidence in schizophrenia, Biol. Psychiatry, 2020, vol. 88, no. 3, p. 273.
Dong, D., Wang, Y., Chang, X., et al., Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity, Schizophr. Bull., 2018, vol. 44, no. 1, p. 168.
Amico, F., O’Hanlon, E., Kraft, D., et al., Functional connectivity anomalies in adolescents with psychotic symptoms, PLoS One., 2017, vol. 12, no. 1. e0169364
Hur, J.W., Kim, T., Cho, K.I.K., and Kwon, J.S., Attenuated resting-state functional anticorrelation between attention and executive control networks in schizotypal personality disorder, J. Clin. Med., 2021, vol. 10, no. 2, p. 312.
Fonseca-Pedrero, E., Lemos-Giraldez, S., Muniz, J., et al., Schizotypy in adolescence: the role of gender and age, J. Nerv. Ment. Dis., 2008, vol. 196, no. 2, p. 161.
Bora, E. and Baysan Arabaci, L., Effect of age and gender on schizotypal personality traits in the normal population, Psychiatry Clin. Neurosci., 2009, v. 63, no. 5, p. 663.
Filippi, M., Valsasina, P., Misci, P., et al., The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects, Hum. Brain. Mapp., 2013, vol. 34, no. 6, p. 1330.
Zhang, C., Cahill, N.D., Arbabshirani, M.R., et al., Sex and age effects of functional connectivity in early adulthood, Brain Connect., 2016, vol. 6, no. 9, p. 700.
Weissman-Fogel, I., Moayedi, M., Taylor, K.S., et al., Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum. Brain Mapp., 2010, vol. 31, no. 11, p. 1713.
ACKNOWLEDGMENTS
The authors are grateful to the radiologists Daria Bazhenova and Anastasiya Suslina.
Funding
The study was supported by the Russian Foundation for Basic Research (RFBR) grant 20-013-00748, a part of the work was supported by the RFBR grant 18-00-01598 (18-00-01592).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All examinations were carried out according to the principles of biomedical ethics and approved by the Interuniversity Ethics Committee of Moscow.
INFORMED CONSENT
Each participant of the study gave a voluntary written informed consent signed after the explanation of potential risks and benefits as well as the details of further examination.
CONFLICT OF INTERESTS
The authors declare no apparent or potential conflicts of interest related to publication of the current article.
IMPACT OF EACH AUTHOR TO THE ARTICLE
All authors wereinvolved in the preparation of the article’s text. Irina S. Lebedeva developed the concept of the study and organized the study. Yana R. Panikratova analyzed the fMRI and SPQ data. Ekaterina V. Pechenkova organized the study and provided guidance on the analysis techniques.va and Anastasiya Suslina.
Rights and permissions
About this article
Cite this article
Lebedeva, I.S., Panikratova, Y.R. & Pechenkova, E.V. Brain Functional Connectivity in Mentally Healthy Individuals with Different Levels of Schizotypy. Hum Physiol 48, 487–495 (2022). https://doi.org/10.1134/S0362119722700013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722700013