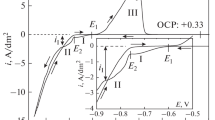
Abstract
The electrochemical behavior of a heavy tungsten alloy W–Ni–Fe ((wt %): 78.8 W, 15.2 Ni, 6.0 Fe) in solutions of sodium hydroxide (2–6 M) and ammonium hydroxide (0–6 M) is studied by linear voltammetry in the potentiodynamic mode, galvanostatic electrolysis, and electrolysis using sinusoidal alternating current (ac) of the industrial frequency. A high rate of alloy dissolution is revealed for both alkaline and ammonia–alkali solutions under the direct current (dc) conditions. When tungsten is etched off from the W–Ni–Fe alloy surface, the rate of its electrochemical oxidation is shown to decrease because of the enrichment of the alloy surface in an Fe–Ni solid solution, which is electrochemically passive in alkaline electrolytes. The addition of ammonium hydroxide to the electrolyte exerts a positive effect on the oxidation. The destructive action of ac on the processed material surface enriched in nickel and iron is elucidated; this action intensifies the subsequent oxidation of the material. Alternative application of dc and ac is found to be reasonable to optimize the main parameters (dissolution rate, current efficiency) of the electrochemical dissolution of the W–Ni–Fe alloys in ammonia–alkali electrolytes.






Similar content being viewed by others
REFERENCES
A. Shemi, A. Magumase, S. Ndlovu, and N. Sacks, “Recycling of tungsten carbide scrap metal: a review of recycling methods and future prospects,” Miner. Eng. 122, 195–205 (2018).
L. Shen, X. Li, D. Linberg, and P. Taskinen, “Tungsten extractive metallurgy: a review of processes and their challenges for sustainability,” Miner. Eng. 142, 105934 (2019).
K. S. S. Kalyan, J. Vimala, Y. Sushma, P. K. Sahoo, and M. Sankaranarayana, “Large scale synthesis of nanocrystalline tungsten powders through recycling of tungsten heavy alloy scrap,” Mater. Today Com. 11, 174–178 (2017).
H. Koohestani, “Characterization of TiO2/WO3 composite produced with recycled WO3 nanoparticles from W–Ni–Fe alloy,” Mater. Chem. Phys. 229, 251–256 (2019).
G. A. Baranov, M. N. Gavrish, and D. D. Sanikovich, “Fabrication of nanosized powders by processing of wastes based on tungsten-containing alloys and study of their granulometric composition,” Vestn. Nats. Tekhn. Univ. Ukr. Kievsk. Politekhn. Institut., Ser. Mashinooborud., No. 63, 42–46 (2011).
V. V. Reznichenko, A. M. Butenko, and O. Ya. Loboiko, “Character of iron recovery from tungsten-containing tool alloys in the presence of ozone,” Trudy Odessk. Politekhn. Univ., No. 2, 205–209 (2009).
S. L. Berezina, V. N. Goryacheva, N. N. Dvulichanskaya, and V. I. Ermolaeva, “Anodic dissolution of a tungsten–zirconium alloy in an alkaline electrolyte,” Vestn. MGTU, Ser. Estestv. Nauki, No. 2, 96–104 (2017).
A. M. Palant, A. M. Levin, and L. A. Palant, “A method for electrochemical processing of metallic wastes of a tungsten–copper alloys,” RF Patent 2479652, Byull. Izobret., No. 11 (2013).
O. G. Kuznetsova, A. M. Levin, M. A. Sevost’yanov, O. I. Tsybin, A. O. Bol’shikh, and M. A. Bol’shikh, “Improved electrodialysis synthesis of perrhenic acid from the electrolytes of processing the wastes of tungsten–rhenium alloys,” Russ. Metall. (Metally), No. 1, 71–76 (2020).
S. Hairunnisha, G. K. Sendil, R. J. Prabhakar, G. N. Srinivasan, P. Adaikkalam, and S. Kulandaisamy, “Studies on the preparation of pure ammonium paratungstate from tungsten alloy scrap,” Hydrometallurgy 85, 67–71 (2007).
O. G. Kuznetsova, A. M. Levin, M. A. Sevost’yanov, O. I. Tsybin, and A. O. Bol’shikh, “Electrochemical oxidation of a heavy tungsten-containing VNZh-type alloy and its components in ammonia–alkali electrolytes,” Russ. Metall. (Metally), No. 5, 507–510 (2019).
V. V. Parshutin, “Corrosion and electrochemical behavior of tungsten-based pseudoalloys and their components,” Elekron. Obrab. Mater., No. 6, 27–45 (2008).
O. G. Kuznetsova, A. M. Levin, M. A. Sevost’yanov, O. I. Tsybin, and A. O. Bolshikh, “Electrochemical recycling of nickel–cobalt-containing tungsten alloys,” IOP Conf. Ser. Mater. Sci. Eng. 525, 012088 (2019).
G. I. Zozulya, O. I. Kuntii, V. M. Srebnii, V. R. Ivashkiv, and V. T. Yavor’s’kii, “A method of electrochemical processing of secondary tungsten scrap,” UA Patent 23360, Byul., No. 7 (2007).
V. Kovalenko and V. Kotoc, “Selective anodic treatment or W(WC)-based superalloy scrap,” Eastern-European J. Enterprise Technol. 85 (1/5), 53–58 (2017).
A. B. Kilimnik and E. Yu. Nikiforova, “Electrochemical behavior of nickel and its oxides in concentrated solutions of sodium hydroxide,” Electrochemistry 12, 1251–1255 (2013).
A. B. Kilimnik and E. Yu. Ostozhkova, Electrochemical Synthesis of Nanodispersed Powders of Metal Oxides (TGTU, Tambov, 2012).
V. R. Guriev, “Study and development of the technology for processing of wastes of heat-resistant and heavy nonferrous metals using electrochemical methods,” Extended Abstract of Doct. Sci. (Eng.) Dissertation (Vladikavkaz, 2001).
A. B. Baeshov, U. A. Abduvalieva, and M. Zh. Zhurinov, “Dissolution of tungsten in an alkaline medium by polarization with nonstationary current,” Izv. Nats. Akad. Nauk Resp. Kazakhstan. Ser. Khim., No. 4, 3–6 (2007).
O. M. Levchuk, “Electrochemical oxidation of wastes of rare heat-resistant metals and their alloys under industrial alternating current,” Extended Abstract of Cand. Sci. (Eng.) Dissertation (Moscow, 2011).
A. T. Vas’ko, Electrochemistry of Heat-Resistant Metals (Tekhnika, Kiev, 1983).
O. G. Kuznetsova, A. M. Levin, M. A. Sevost’yanov, “Electrochemical properties of phase components of the VNZh alloy in ammonia–alkali electrolytes,” in Proceedings of XI International Conference on Modern Methods in Theoretical and Experimental Electrochemistry (Inst. Khimii Rastvorov, Ples, 2020), p. 94.
Phase Diagram of Binary Metallic Systems: A Handbook Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 1997), Vol. 3.
Funding
This work was performed in terms of state assignment no. 075-00746-19-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Kuznetsova, O.G., Levin, A.M., Sevost’yanov, M.A. et al. Electrochemical Processing of a Heavy W–Ni–Fe Alloy by Direct and Alternating Currents in Ammonia–Alkali Solutions. Russ. Metall. 2021, 586–593 (2021). https://doi.org/10.1134/S0036029521050104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521050104