Abstract
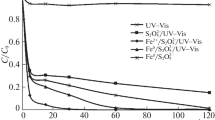
The kinetic regularities of photochemical oxidation of stable complex cyanides (hexacyanoferrates) with persulfate (oxidizing system {UV/S2O\(_{8}^{{2 - }}\)}) and hydrogen peroxide (oxidizing system {UV/H2O2}) under the influence of quasi-monochromatic UVC radiation from a KrCl excilamp (222 nm) have been studied. According to the efficiency and rate of the destruction of the target compound, the oxidizing systems under study can be arranged in the following series: {UV/S2O\(_{8}^{{2 - }}\)} > {UV/H2O2} > {UV}. The effective destruction of hexacyanoferrates at micromolar concentrations (≤47 μM) to nontoxic and biodegradable compounds in the combined {UV/S2O\(_{8}^{{2 - }}\)} system is due to the high oxidizing ability of reactive oxygen species formed as a result of persulfate photolysis.





Similar content being viewed by others
REFERENCES
Y. Deng and R. Zhao, Curr. Pollut. Rep. 1, 167 (2015). https://doi.org/10.1007/s40726-015-0015-z
S. Giannakis, K. Y. A. Lin, and F. Ghanbari, Chem. Eng. J. 406 (2021). https://doi.org/10.1016/j.cej.2020.127083
O. M. Rodriguez-Narvaez, J. M. Peralta-Hernez, A. Goonetilleke, et al., Chem. Eng. J. 323, 361 (2017). https://doi.org/10.1016/j.cej.2017.04.106
Y. Yang, Y. S. Ok, K.-H. Kim, et al., Sci. Total Environ. 596–597, 303 (2017). https://doi.org/10.1016/j.scitotenv.2017.04.102
Q. Yang, Y. Ma, F. Chen, et al., Chem. Eng. J. 378, 122149 (2019). https://doi.org/10.1016/j.cej.2019.122149
W. Huang, A. Bianco, M. Brigante, et al., J. Hazard. Mater. 347, 279 (2018). https://doi.org/10.1016/j.jhazmat.2018.01.006
S. Malato, P. Fernez-Ibanez, M. Maldonado, et al., Catal. Today 147, 1 (2009). https://doi.org/10.1016/j.cattod.2009.06.018
O. Tsydenova, V. Batoev, and A. Batoeva, Int. J. Environ. Res. Publ. Health 12, 9542 (2015). https://doi.org/10.3390/ijerph120809542
A. M. Boichenko, M. I. Lomaev, A. N. Panchenko, et al., Ultraviolet and Vacuum-Ultraviolet Excilamps: Physics, Technology, and Applications (STT, Tomsk, 2011) [in Russian].
E. Sosnin, S. Avdeev, V. Tarasenko, V. S. Skakun, and D. V. Schitz, Instrum. Exp. Tech. 58, 309 (2015). https://doi.org/10.1134/S0020441215030124
S. Popova, G. Matafonova, and V. Batoev, Ecotoxicol. Environ. Saf. 169, 169 (2019). https://doi.org/10.1016/j.ecoenv.2018.11.014
M. Sizykh, A. Batoeva, and O. Tsydenova, Clean-Soil, Air, Water. 46, 1700187 (2018). https://doi.org/10.1002/clen.201700187
M. Sizykh, A. Batoeva, and G. Matafonova, J. Photochem. Photobiol., A 436, 114357 (2023). https://doi.org/10.1016/j.jphotochem.2022.114357
G. Matafonova and V. Batoev, Chemosphere 89, 637 (2012). https://doi.org/10.1016/j.chemosphere.2012.06.012
S. L. Budaev, A. A. Batoeva, M. S. Khandarkhaeva, and D. G. Aseev, Russ. J. Phys. Chem. A 91, 604 (2017). https://doi.org/10.1134/S0036024417030049
M. M. Botz, T. I. Mudder, and A. U. Akcil, in Gold Ore Processing, Ed. by M. D. Adams (Elsevier, Amsterdam, 2016), Chap. 35, p. 619. https://doi.org/10.1016/B978-0-444-63658-4.00035-9
N. Kuyucak and A. Akcil, Miner. Eng. 50–51, 13 (2013). https://doi.org/10.1016/j.mineng.2013.05.027
S. Canonica, L. Meunier, and U. von Gunten, Water Res. 42, 121 (2008). https://doi.org/10.1016/j.watres.2007.07.026
PND F 14.1: 2.164-2000: Quantitative chemical analysis of water. Methodology for measuring mass concentrations of hexacyanoferrates in samples of natural and waste waters using the photometric method (2009).
PND F 14.1: 2.56-96: Quantitative chemical analysis of water. Method for measuring the mass concentration of cyanide in natural and waste waters using the photometric method with pyridine and barbituric acid (2015).
J. Yang, M. Zhu, and D. D. Dionysiou, Water Res. 189, 116627 (2021). https://doi.org/10.1016/j.watres.2020.116627
F. L. Rosario-Ortiz, E. C. Wert, and S. A. Snyder, Water Res. 44, 1440 (2010). https://doi.org/10.1016/j.watres.2009.10.031
J. Sharma, I. M. Mishra, and V. Kumar, J. Environ. Manage. 156, 266 (2015). https://doi.org/10.1016/j.jenvman.2015.03.048
S. Yang, P. Wang, X. Yang, et al., J. Hazard. Mater. 179, 552 (2010). https://doi.org/10.1016/j.jhazmat.2010.03.039
P. G. Anipsitakis and D. Dionysiou, Appl. Catal. B 54, 155 (2004). https://doi.org/10.1016/j.apcatb.2004.05.025
F. Ghanbari and M. Moradi, Chem. Eng. J. 310 (2017). https://doi.org/10.1016/j.cej.2016.10.064
O. S. Furman, A. L. Teel, and R. J. Watts, Environ. Sci. Technol. 44, 6423 (2010). https://doi.org/10.1021/es1013714
H. Kusic, I. Peternel, S. Ukic, et al., Chem. Eng. J. 172, 109 (2011). https://doi.org/10.1016/j.cej.2011.05.076
P. Neta, R. Huie, and B. A. Ross, J. Phys. Chem. Ref. Data 17, 1027 (1988). https://doi.org/10.1063/1.555808
H. Ibargüen-López, B. López-Balanta, L. Betancourt-Buitrago, et al., J. Environ. Chem. Eng. 9, 106233 (2021). https://doi.org/10.1016/j.jece.2021.106233
X. Duan, X. Niu, J. Gao, et al., Curr. Opin. Chem. Eng. 38, 100867 (2022). https://doi.org/10.1016/j.coche.2022.100867
Y.-M. Lee, G. Lee, and K.-D. Zoh, J. Hazard. Mater. 403, 123591 (2021). https://doi.org/10.1016/j.jhazmat.2020.123591
L. C. Clifton and R. Huie, Int. J. Chem. Kinet. 21, 677 (1989). https://doi.org/10.1002/kin.550210807
G. V. Buxton, C. L. Greenstock, W. P. Helman, et al., J. Phys. Chem. Ref. Data 17, 513 (1988). https://doi.org/10.1063/1.555805
S.-N. Nam, S.-K. Han, J.-W. Kang, et al., Ultrason. Sonochem. 10, 139 (2003). https://doi.org/10.1016/S1350-4177(03)00085-3
S. A. Popova, G. G. Matafonova, and V. B. Batoev, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 62, 118 (2019). https://doi.org/10.6060/ivkkt.20196205.5819
V. A. Svetlichnyi, R. T. Kuznetsova, T. N. Kopylova, et al., Opt. Atmos. Okeana 14, 38 (2001).
C. Chen, Y. Du, Y. Zhou, et al., Water Res. 194, 116914 (2021). https://doi.org/10.1016/j.watres.2021.116914
B. Sun, Y. Zheng, C. Shang, et al., J. Hazard. Mater. 430, 128450 (2022). https://doi.org/10.1016/j.jhazmat.2022.128450
Funding
This study was performed under the Basic Research Program (no. FWSU-2021-0006) of the Baikal Institute of Nature Management (BINM), Siberian Branch, Russian Academy of Sciences using the equipment of the Multiaccess Center of BINM (CCU BINM SB RAS (Ulan-Ude, Russia)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Batoeva, A.A., Tsybikova, B.A. & Sizykh, M.R. Photoinduced Destruction of Complex Cyanides Using Quasi-Monochromatic UVC Radiation of a KrCl Excilamp (222 nm). Russ. J. Phys. Chem. 97, 2855–2860 (2023). https://doi.org/10.1134/S003602442312004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442312004X